Korean Diabetes J.
2009 Dec;33(6):458-463. 10.4093/kdj.2009.33.6.458.
Stimulation of Glucagon Like Peptide-1 Secretion in Enteroendocrine L cells
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Konyang University School of Medicine, Daejeon, Korea.
- KMID: 2298050
- DOI: http://doi.org/10.4093/kdj.2009.33.6.458
Abstract
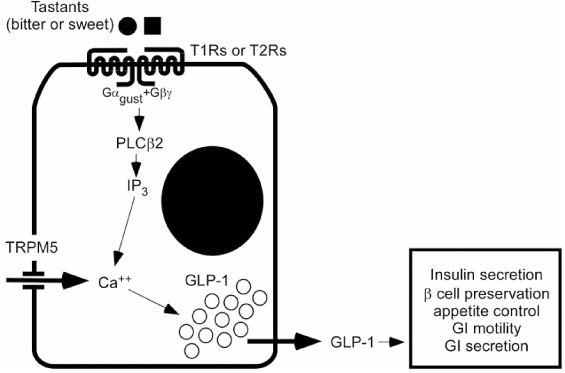
- GLP-1 (glucagon like peptide-1) is new anti-diabetic drug with a number of beneficial effects. It stimulates glucose dependant insulin secretion and restoration of beta cell mass through enhancement of islet mass. However, it is easily inactivated after being secreted from enteroendocrine L cells. Recent trial to increased GLP-1 is to directly stimulate L cells through its receptor located in the surface of L cell. Taste receptor in the apical surface of L cell is activated by various tastants contained in the food. Tongue perceives taste sense through the heterotrimeric G-protein (alpha-gustducin) and its downstream signaling cascades. Same taste receptors are also expressed in enteroendocrine cells. In duodenal L cell, alpha-gustducin was detected by immunofluorescence stainig at the luminal projections of enteroendocrine cells. And several other taste signaling elements were also found in L cells. Ingestion of sweet or bitter compounds revealed stimulation of GLP-1 secretion and the regulation of plasma insulin and glucose. In this review, I will briefly introduce the possibilities to stimulate GLP-1 secretion though the membrane receptor in enteroendocrine cell. And it will be the good candidate to develop the treatment modality for obesity, diabetes and abnormal gut motility.
MeSH Terms
Figure
Cited by 1 articles
-
Exendin-4 Protects Oxidative Stress-Induced β-Cell Apoptosis through Reduced JNK and GSK3β Activity
Ju-Young Kim, Dong-Mee Lim, Chan Il Moon, Kyung-Jin Jo, Seong-Kyu Lee, Haing-Woon Baik, Ki-Ho Lee, Kang-Woo Lee, Keun-Young Park, Byung-Joon Kim
J Korean Med Sci. 2010;25(11):1626-1632. doi: 10.3346/jkms.2010.25.11.1626.
Reference
-
1. Doyle ME, Egan JM. Glucagon-like peptide-1. Recent Prog Horm Res. 2001. 56:377–399.2. Nauck MA, Holst JJ, Willms B, Schmiegel W. Glucagon-like peptide 1 (GLP-1) as a new therapeutic approach for type 2-diabetes. Exp Clin Endocrinol Diabetes. 1997. 105:187–195.3. Knop FK, Vilsboll T. Gglp-1-based treatment of type 2 diabetes mellitus. Ugeskr Laeger. 2007. 169:2095–2099.4. Buchan AM. Nutrient tasting and signaling mechanisms in the gut III. Endocrine cell recognition of luminal nutrients. Am J Physiol. 1999. 277:G1103–G1107.5. Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A g protein-coupled receptor responsive to bile acids. J Biol Chem. 2003. 278:9435–9440.6. Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002. 298:714–719.7. Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005. 329:386–390.8. Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R. Anti-hyperglycemic activity of a TGR5 agonist isolated from olea europaea. Biochem Biophys Res Commun. 2007. 362:793–798.9. Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-x-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009. 30:570–580.10. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. Tgr5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009. 10:167–177.11. Tiwari A, Maiti P. Tgr5: an emerging bile acid g-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov Today. 2009. 14:523–530.12. Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005. 11:90–94.13. Gotoh C, Hong YH, Iga T, Hishikawa D, Suzuki Y, Song SH, Choi KC, Adachi T, Hirasawa A, Tsujimoto G, Sasaki S, Roh SG. The regulation of adipogenesis through GPR120. Biochem Biophys Res Commun. 2007. 354:591–597.14. Katsuma S, Hatae N, Yano T, Ruike Y, Kimura M, Hirasawa A, Tsujimoto G. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J Biol Chem. 2005. 280:19507–19515.15. Ruiz-Avila L, McLaughlin SK, Wildman D, McKinnon PJ, Robichon A, Spickofsky N, Margolskee RF. Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature. 1995. 376:80–85.16. Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000. 100:693–702.17. Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000. 404:601–604.18. Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002. 277:1–4.19. Raybould HE. Does your gut taste? Sensory transduction in the gastrointestinal tract. News Physiol Sci. 1998. 13:275–280.20. Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999. 277:G922–G928.21. Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in l cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006. 291:G792–G802.22. Rozengurt E, Sternini C. Taste receptor signaling in the mammalian gut. Curr Opin Pharmacol. 2007. 7:557–562.23. Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007. 104:15069–15074.24. Egan JM, Margolskee RF. Taste cells of the gut and gastrointestinal chemosensation. Mol Interv. 2008. 8:78–81.25. Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A. 2002. 99:2392–2397.26. Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1r3 and gustducin in gut sense sugars to regulate expression of na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007. 104:15075–15080.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of the Sweet Taste Receptor in Enteroendocrine Cells and Pancreatic beta-Cells
- Extrapancreatic Effect of Glucagon like Peptide-1
- Clinical Application of Glucagon-Like Peptide-1 Receptor Agonists
- Pleiotropic Effects of an Incretin Hormone
- Ca2+ entry through reverse Na+ /Ca2+ exchanger in NCI-H716, glucagon-like peptide-1 secreting cells