Korean Circ J.
2009 Feb;39(2):75-78. 10.4070/kcj.2009.39.2.75.
Abciximab (ReoPro)-Induced Thrombocytopenia Diagnosed Through Measurement of Heparin-Dependent Antibody
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea. thpark65@dau.ac.kr
- KMID: 2297953
- DOI: http://doi.org/10.4070/kcj.2009.39.2.75
Abstract
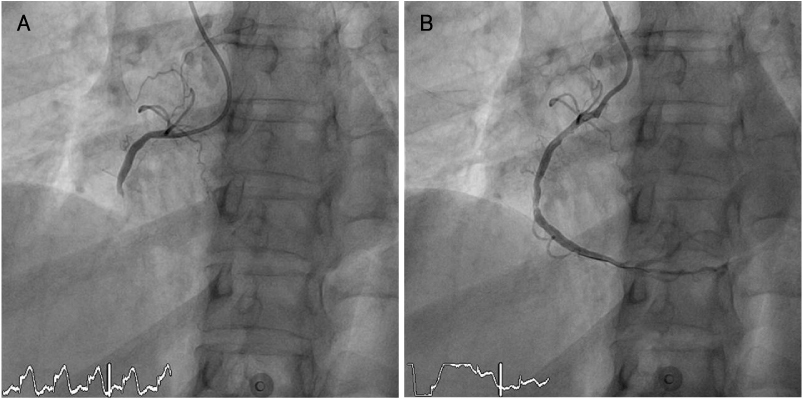
- Abciximab (ReoPro) is an extremely potent inhibitor of the glycoprotein IIb/IIIa receptor. Its main application is in the maintenance of coronary flow after suboptimal coronary intervention. Complications associated with this drug include bleeding and severe thrombocytopenia. We report a case of severe thrombocytopenia secondary to abciximab therapy for percutaneous coronary intervention in a 65-year-old woman suffering from an acute myocardial infarction. Her platelet count dropped to 1,000/mm3 7 hours after abciximab administration and improved with transfusion of 12 units of platelet concentrate the following day. The patient was diagnosed through measurement of heparin-dependent antibodies and readministration of heparin.
Keyword
MeSH Terms
-
Aged
Antibodies
Antibodies, Monoclonal
Blood Platelets
Female
Glycoproteins
Hemorrhage
Heparin
Humans
Immunoglobulin Fab Fragments
Myocardial Infarction
Percutaneous Coronary Intervention
Platelet Count
Stress, Psychological
Thrombocytopenia
Antibodies
Antibodies, Monoclonal
Glycoproteins
Heparin
Immunoglobulin Fab Fragments
Figure
Reference
-
1. Coller BS. Blockade of platelet GPIIb/IIIa receptors as an antithrombotic strategy. Circulation. 1995. 92:2373–2380.2. Topol EJ, Byzova TV, Plow EF. Platelet GP IIb-IIIa blockers. Lancet. 1999. 353:227–231.3. Kereiakes DJ, Berkowitz SD, Lincoff AM, et al. Clinical correlates and course of thrombocytopenia during percutaneous coronary intervention in the era of abciximab platelet glycoprotein IIb/IIIa blockade. Am Heart J. 2000. 140:74–80.4. Berkowitz SD, Harrington RA, Rund MM, Tcheng JE. Acute profound thrombocytopenia after c7E3 Fab (abciximab) therapy. Circulation. 1997. 95:809–813.5. Aster RH, Curtis BR, Bougie DW, et al. Thrombocytopenia associated with the use of GPIIb/IIIa inhibitors: position paper of the ISTH working group on thrombocytopenia and GPIIb/IIIa inhibitors. J Thromb Haemost. 2006. 4:678–679.6. The EPIC Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994. 330:956–961.7. The CAPTURE Investigators. Randomized placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina. Lancet. 1997. 349:1429–1435.8. The EPILOG Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. N Engl J Med. 1997. 336:1689–1696.9. The EPISTENT Investigators. Randomized placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein IIb/IIIa blockade. Lancet. 1998. 352:87–92.10. Kim W, Jeong MH, Kim KH, et al. The rescue use of a platelet glycoprotein IIb/IIIa receptor blocker (Abciximab; Reo-pro®) in high-risk patients with acute myocardial infarction underwent percutaneous coronary intervention. Korean Circ J. 2001. 31:492–499.11. Butler R, Hubner PJ. Acute severe thrombocytopenia after treatment with abciximab. Heart. 2000. 83:e5.12. Coller BS. Anti-GP IIb/IIIa drugs: current strategies and future directions. Thomb Haemost. 2001. 86:427–443.13. Madan M, Kereiakes D, Hermiller J, et al. Efficacy of abciximab readministration in coronary intervention. Am J Cardiol. 2000. 85:435–440.14. Merlini PA, Rossi M, Menozzi A, et al. Thrombocytopenia caused by abciximab or tirofiban and its association with clinical outcome in patients undergoing coronary stenting. Circulation. 2004. 109:2203–2206.15. Weimann G, Lubenow N, Selleng K, Eichler P, Albrecht D, Greinacher A. Glucosamine sulfate dose not cross-react with the antibodies of patients with heparin-induced thrombocytopenia. Br J Haematol. 2001. 66:195–199.16. Aster RH, Curtis BR, Bougie DW, et al. Thrombocytopenia associated with the use of GP IIb/IIIa inhibitors: position paper of the ISTH working group on thrombocytopenia and GP IIb/IIIa inhibitors. J Thromb Haemost. 2006. 4:678–679.17. Kelton JG, Sheridan D, Brain H, Powers PJ, Turpie AG, Carter CJ. Clinical usefulness of testing for a heparin-dependent platelet-aggregating factor in patients with suspected heparin-associated thrombocytopenia. J Lab Clin Med. 1984. 103:606–612.18. Sane DC, Damaraju LV, Topol EJ, et al. Occurrence and clinical significance of pseudothrombocytopenia during abciximab therapy. J Am Coll Cardiol. 2000. 36:75–83.19. Ferguson JJ, Kereiakes DJ, Adgey AA, et al. Safe use of platelet GP IIb/IIIa inhibitors. Am Heart J. 1998. 135:S77–S89.20. Kim MH, Jung ES, Yoon SG, et al. A case of abciximab induced acute profound thrombocytopenia resulting in compartment syndrome of the forearm from massive bleeding at the brachial artery access site. Korean Circ J. 2007. 37:594–598.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Heparin-Induced Thrombocytopenia Diagnosed by Particle Gel Immunoassay
- Cardiopulmonary Bypass in Patient with Heparin-Induced Thrombocytopenia Employing Recombinant Hirudin
- Heparin-induced Thrombocytopenia Type II after Free Flap Operation
- A case of acute profound thrombocytopenia following abciximab therapy
- Two Cases of Heparin-Induced Thrombocytopenia in Hemodialysis Patients