Korean Circ J.
2015 Mar;45(2):149-157. 10.4070/kcj.2015.45.2.149.
Cervical Vagal Nerve Stimulation Activates the Stellate Ganglion in Ambulatory Dogs
- Affiliations
-
- 1Krannert Institute of Cardiology and Division of Cardiology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA. chenpp@iupui.edu
- 2Department of Neurology, Indiana University School of Medicine, Indianapolis, IN, USA.
- 3Department of Pathology and Laboratory Medicine, The David Geffen School of Medicine, UCLA, Los Angeles, CA, USA.
- 4Department of Internal Medicine, Chonbuk National University School of Medicine, Jeonju, Korea.
- 5Department of Cardiovascular Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 6Division of Cardiology, College of Medicine, Catholic University of Daegu, Daegu, Korea.
- KMID: 2297882
- DOI: http://doi.org/10.4070/kcj.2015.45.2.149
Abstract
- BACKGROUND AND OBJECTIVES
Recent studies showed that, in addition to parasympathetic nerves, cervical vagal nerves contained significant sympathetic nerves. We hypothesized that cervical vagal nerve stimulation (VNS) may capture the sympathetic nerves within the vagal nerve and activate the stellate ganglion.
MATERIALS AND METHODS
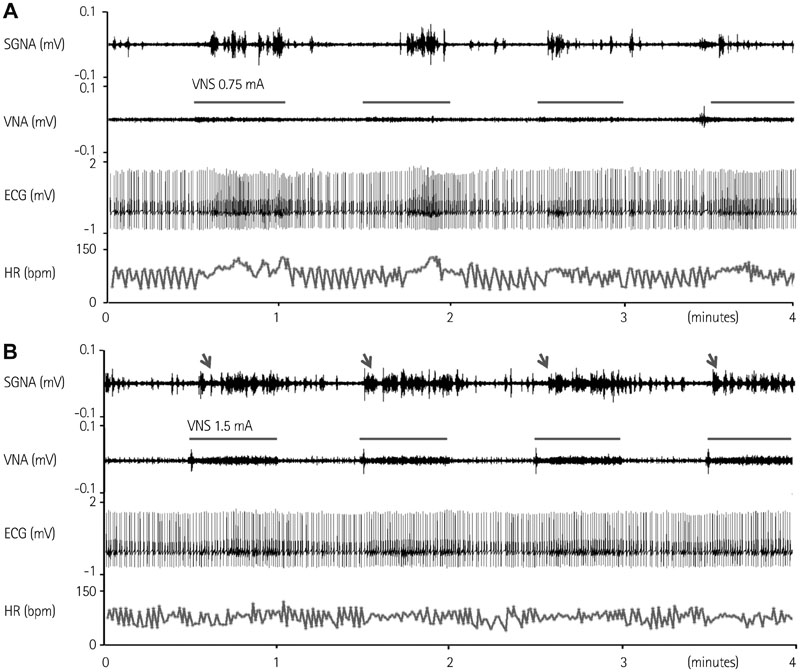
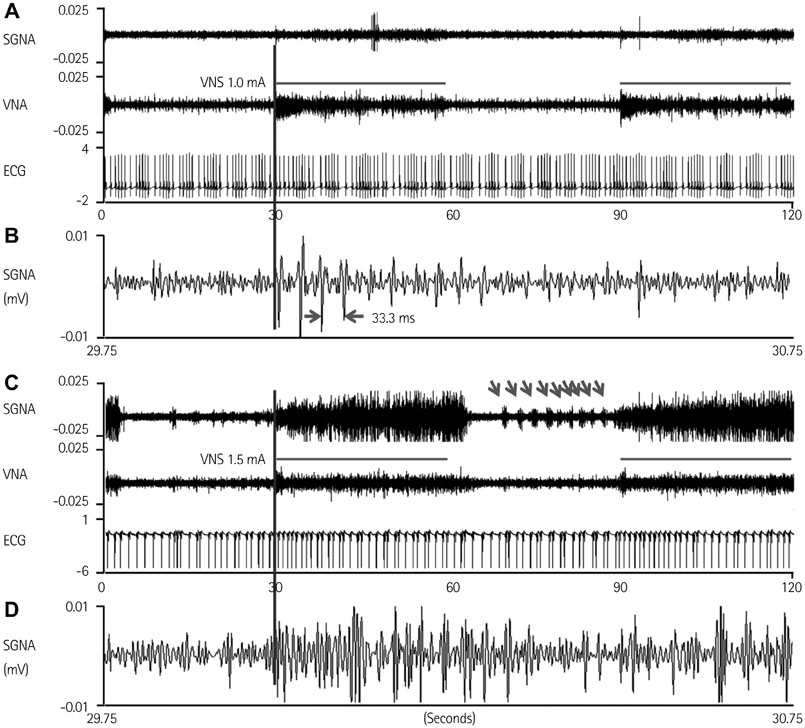
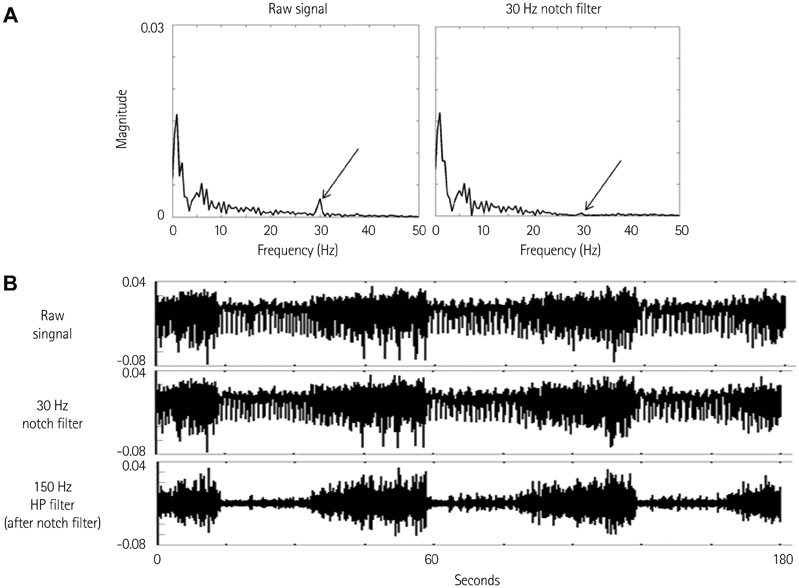
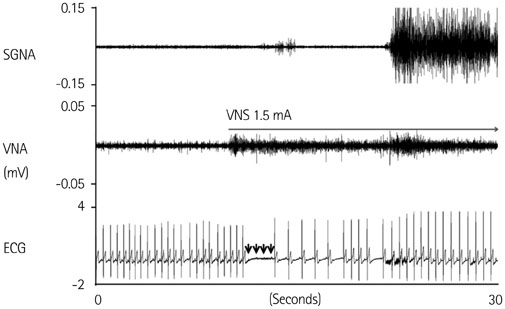
We recorded left stellate ganglion nerve activity (SGNA), left thoracic vagal nerve activity (VNA), and subcutaneous electrocardiogram in seven dogs during left cervical VNS with 30 seconds on-time and 30 seconds off time. We then compared the SGNA between VNS on and off times.
RESULTS
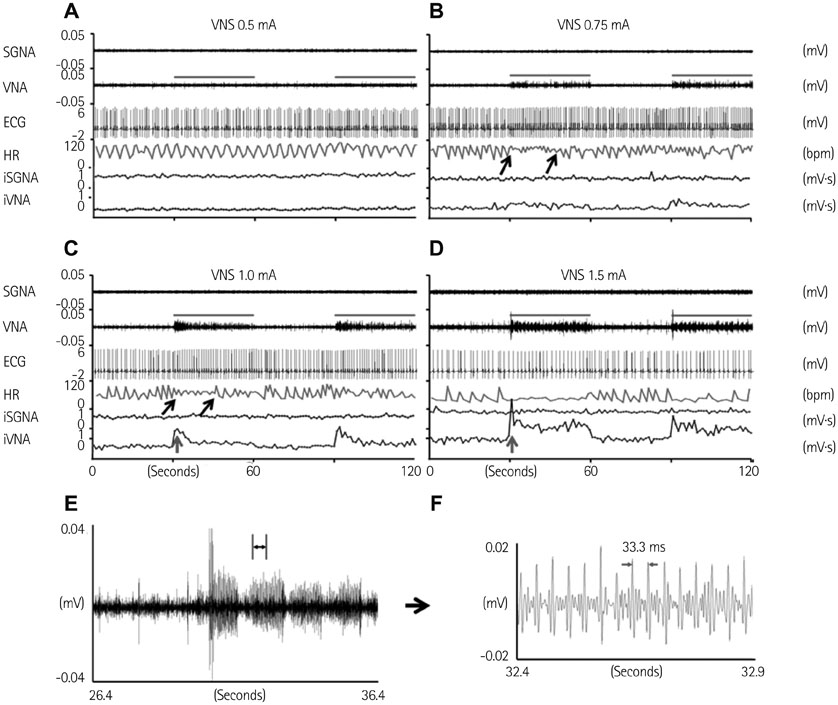
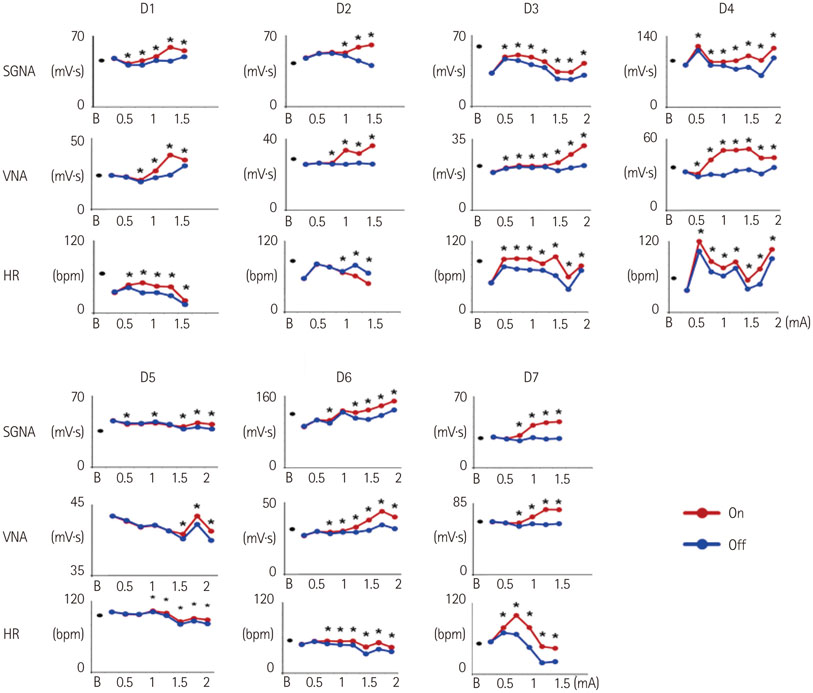
Cervical VNS at moderate (0.75 mA) output induced large SGNA, elevated heart rate (HR), and reduced HR variability, suggesting sympathetic activation. Further increase of the VNS output to >1.5 mA increased SGNA but did not significantly increase the HR, suggesting simultaneous sympathetic and parasympathetic activation. The differences of integrated SGNA and integrated VNA between VNS on and off times (DeltaSGNA) increased progressively from 5.2 mV-s {95% confidence interval (CI): 1.25-9.06, p=0.018, n=7} at 1.0 mA to 13.7 mV-s (CI: 5.97-21.43, p=0.005, n=7) at 1.5 mA. The difference in HR (DeltaHR, bpm) between on and off times was 5.8 bpm (CI: 0.28-11.29, p=0.042, n=7) at 1.0 mA and 5.3 bpm (CI 1.92 to 12.61, p=0.122, n=7) at 1.5 mA.
CONCLUSION
Intermittent cervical VNS may selectively capture the sympathetic components of the vagal nerve and excite the stellate ganglion at moderate output. Increasing the output may result in simultaneously sympathetic and parasympathetic capture.
MeSH Terms
Figure
Reference
-
1. Terry R. Vagus nerve stimulation: a proven therapy for treatment of epilepsy strives to improve efficacy and expand applications. Conf Proc IEEE Eng Med Biol Soc. 2009; 2009:4631–4634.2. Premchand RK, Sharma K, Mittal S, et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail. 2014; 20:808–816.3. Zannad F, De Ferrari GM, Tuinenburg AE, et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the neural cardiac therapy for heart failure (NECTAR-HF) randomized controlled trial. Eur Heart J. 2014; 36:425–433.4. De Ferrari GM, Crijns HJ, Borggrefe M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011; 32:847–855.5. Klein HU, Ferrari GM. Vagus nerve stimulation: a new approach to reduce heart failure. Cardiol J. 2010; 17:638–644.6. Sabbah HN. Electrical vagus nerve stimulation for the treatment of chronic heart failure. Cleve Clin J Med. 2011; 78:Suppl 1. S24–S29.7. Onkka P, Maskoun W, Rhee KS, et al. Sympathetic nerve fibers and ganglia in canine cervical vagus nerves: localization and quantitation. Heart Rhythm. 2013; 10:585–591.8. Kawagishi K, Fukushima N, Yokouchi K, Sumitomo N, Kakegawa A, Moriizumi T. Tyrosine hydroxylase-immunoreactive fibers in the human vagus nerve. J Clin Neurosci. 2008; 15:1023–1026.9. Seki A, Green HR, Lee TD, et al. Sympathetic nerve fibers in human cervical and thoracic vagus nerves. Heart Rhythm. 2014; 11:1411–1417.10. Schwartz PJ, Pagani M, Lombardi F, Malliani A, Brown AM. A cardiocardiac sympathovagal reflex in the cat. Circ Res. 1973; 32:215–220.11. Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011; 123:2204–2212.12. Shen MJ, Hao-Che Chang, Park HW, et al. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm. 2013; 10:910–915.13. Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. 2012; 74:245–269.14. Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007; 50:335–343.15. Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008; 118:916–925.16. Robinson EA, Rhee KS, Doytchinova A, et al. Estimating sympathetic tone by recording subcutaneous nerve activity in ambulatory dogs. J Cardiovasc Electrophysiol. 2015; 26:70–78.17. Mizeres NJ. The anatomy of the autonomic nervous system in the dog. Am J Anat. 1955; 96:285–318.18. Ellison JP, Williams TH. Sympathetic nerve pathways to the human heart, and their variations. Am J Anat. 1969; 124:149–162.19. Armour JA, Hageman GR, Randall WC. Arrhythmias induced by local cardiac nerve stimulation. Am J Physiol. 1972; 223:1068–1075.20. Dicarlo L, Libbus I, Amurthur B, Kenknight BH, Anand IS. Autonomic regulation therapy for the improvement of left ventricular function and heart failure symptoms: the ANTHEM-HF study. J Card Fail. 2013; 19:655–660.21. Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003; 107:2355–2360.22. Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005; 2:624–631.23. Goldberger AL, Pavelec RS. Vagally-mediated atrial fibrillation in dogs: conversion with bretylium tosylate. Int J Cardiol. 1986; 13:47–55.24. del Negro CA, Hsiao CF, Chandler SH. Outward currents influencing bursting dynamics in guinea pig trigeminal motoneurons. J Neurophysiol. 1999; 81:1478–1485.25. Köhler M, Hirschberg B, Bond CT, et al. Small-conductance, calciumactivated potassium channels from mammalian brain. Science. 1996; 273:1709–1714.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experimental Study for Innervation of Scalp and Face with WGA-HRP Method
- Treatment of Nerve Root Injury during Cervical Epidural Block: A case report
- Clinical Experiences of Causalgia: Two cases report
- Percutaneous Radiofrequency Thermocoagulation of the Stellate Ganglion in the Treatment of Cervical and Upper Extremity Pain: A case report
- Bilateral Horner's Syndrome after a Stellate Ganglion Block