J Gynecol Oncol.
2013 Apr;24(2):146-153. 10.3802/jgo.2013.24.2.146.
Risk stratification of abdominopelvic failure for FIGO stage III epithelial ovarian cancer patients: implications for adjuvant radiotherapy
- Affiliations
-
- 1Department of Radiation Oncology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. ybkim3@yuhs.ac
- 2Department of Obstetrics and Gynecology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2288546
- DOI: http://doi.org/10.3802/jgo.2013.24.2.146
Abstract
OBJECTIVE
To analyze patterns of abdominopelvic failures and to define subgroups for the use of adjuvant radiotherapy in the International Federation of Gynecology and Obstetrics (FIGO) stage III epithelial ovarian cancer (EOC).
METHODS
We reviewed 149 patients treated with debulking surgery followed by intravenous taxane and platinum chemotherapy between 1999 and 2008. Patient characteristics, patterns of failure, abdominopelvic failure APF-free survival (APFFS) and overall survival (OS) were analyzed.
RESULTS
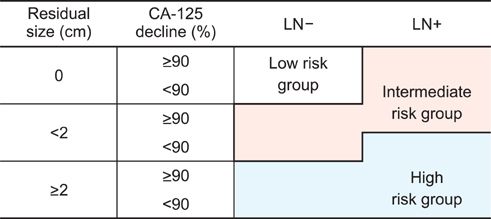
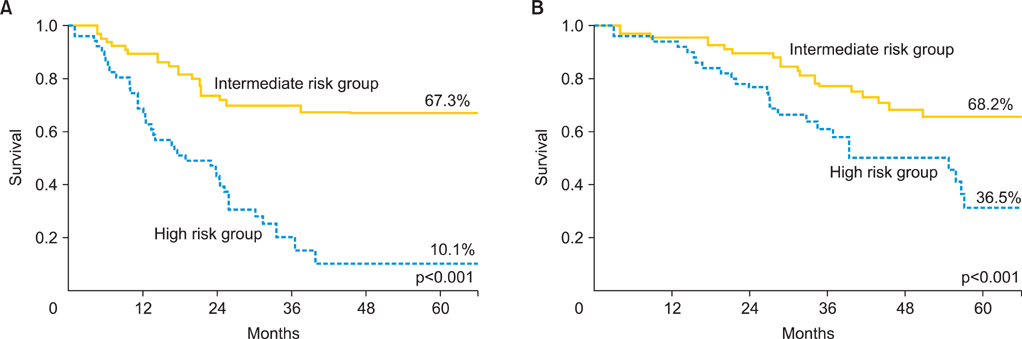
The median age of the patients was 51 years. Thirty-two patients (21.5%) were found to have residuum >2 cm after surgery. The median pretreatment CA-125 was 604 and 54.4% of patients had a decline in CA-125 > or =90% between pretreatment and at postoperative 1 month. With a median follow-up of 50 months, 79 patients (53.0%) experienced abdominopelvic failure (APF). The 5-year APF-free survival rate was 41.1%. Lymph node metastasis, size of residual disease, and decline in CA-125 were found to be significant prognostic factors for APF upon multivariate analysis. The group of patients in whom abdominopelvic irradiation was indicated as definitive postoperative treatment comprised 55% of the overall patient population and their 5-year survival rate was 68%.
CONCLUSION
The stratification was suggested to predict APF based on lymph node metastasis, size of residual tumor, and decline in CA-125. Adjuvant radiotherapy covering the whole abdominopelvis using the intensity modulation technique may be considered to reduce APF in FIGO stage III EOC patients with intermediate risk.
Keyword
MeSH Terms
-
Bridged Compounds
Follow-Up Studies
Gynecology
Humans
Lymph Nodes
Multivariate Analysis
Neoplasm Metastasis
Neoplasm, Residual
Neoplasms, Glandular and Epithelial
Obstetrics
Ovarian Neoplasms
Platinum
Radiotherapy, Adjuvant
Survival Rate
Taxoids
Bridged Compounds
Neoplasms, Glandular and Epithelial
Ovarian Neoplasms
Platinum
Taxoids
Figure
Reference
-
1. McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996. 334:1–6.2. Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2000. 18:106–115.3. Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000. 92:699–708.4. Pectasides D, Pectasides E. Maintenance or consolidation therapy in advanced ovarian cancer. Oncology. 2006. 70:315–324.5. Dembo AJ. Radiotherapeutic management of ovarian cancer. Semin Oncol. 1984. 11:238–250.6. Jaaback K, Johnson N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2006. (1):CD005340.7. Hess LM, Benham-Hutchins M, Herzog TJ, Hsu CH, Malone DC, Skrepnek GH, et al. A meta-analysis of the efficacy of intraperitoneal cisplatin for the front-line treatment of ovarian cancer. Int J Gynecol Cancer. 2007. 17:561–570.8. Sorbe B. Swedish-Norwegian Ovarian Cancer Study Group. Consolidation treatment of advanced (FIGO stage III) ovarian carcinoma in complete surgical remission after induction chemotherapy: a randomized, controlled, clinical trial comparing whole abdominal radiotherapy, chemotherapy, and no further treatment. Int J Gynecol Cancer. 2003. 13:278–286.9. Cardenes H, Randall ME. Integrating radiation therapy in the curative management of ovarian cancer: current issues and future directions. Semin Radiat Oncol. 2000. 10:61–70.10. Barakat RR, Markman M, Randall ME. Principles and practice of gynecologic oncology. 2009. 5th ed. Philadelphia: Lippincott Williams & Wilkins.11. Dembo AJ. Abdominopelvic radiotherapy in ovarian cancer: a 10-year experience. Cancer. 1985. 55:9 Suppl. 2285–2290.12. Goldberg N, Peschel RE. Postoperative abdominopelvic radiation therapy for ovarian cancer. Int J Radiat Oncol Biol Phys. 1988. 14:425–429.13. Carey MS, Dembo AJ, Simm JE, Fyles AW, Treger T, Bush RS. Testing the validity of a prognostic classification in patients with surgically optimal ovarian carcinoma: a 15-year review. Int J Gynecol Cancer. 1993. 3:24–35.14. Dinniwell R, Lock M, Pintilie M, Fyles A, Laframboise S, Depetrillo D, et al. Consolidative abdominopelvic radiotherapy after surgery and carboplatin/paclitaxel chemotherapy for epithelial ovarian cancer. Int J Radiat Oncol Biol Phys. 2005. 62:104–110.15. Rubin SC, Randall TC, Armstrong KA, Chi DS, Hoskins WJ. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol. 1999. 93:21–24.16. Greer BE, Bundy BN, Ozols RF, Fowler JM, Clarke-Pearson D, Burger RA, et al. Implications of second-look laparotomy in the context of optimally resected stage III ovarian cancer: a non-randomized comparison using an explanatory analysis: a Gynecologic Oncology Group study. Gynecol Oncol. 2005. 99:71–79.17. Chung HH, Kang WJ, Kim JW, Park NH, Song YS, Chung JK, et al. Role of [18F]FDG PET/CT in the assessment of suspected recurrent ovarian cancer: correlation with clinical or histological findings. Eur J Nucl Med Mol Imaging. 2007. 34:480–486.18. Sironi S, Messa C, Mangili G, Zangheri B, Aletti G, Garavaglia E, et al. Integrated FDG PET/CT in patients with persistent ovarian cancer: correlation with histologic findings. Radiology. 2004. 233:433–440.19. Pan HS, Lee SL, Huang LW, Chen YK. Combined positron emission tomography-computed tomography and tumor markers for detecting recurrent ovarian cancer. Arch Gynecol Obstet. 2011. 283:335–341.20. Rochet N, Sterzing F, Jensen A, Dinkel J, Herfarth K, Schubert K, et al. Helical tomotherapy as a new treatment technique for whole abdominal irradiation. Strahlenther Onkol. 2008. 184:145–149.21. Kim YB, Kim JH, Jeong KK, Seong J, Suh CO, Kim GE. Dosimetric comparisons of three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and helical tomotherapy in whole abdominopelvic radiotherapy for gynecologic malignancy. Technol Cancer Res Treat. 2009. 8:369–377.22. Rochet N, Sterzing F, Jensen AD, Dinkel J, Herfarth KK, Schubert K, et al. Intensity-modulated whole abdominal radiotherapy after surgery and carboplatin/taxane chemotherapy for advanced ovarian cancer: phase I study. Int J Radiat Oncol Biol Phys. 2010. 76:1382–1389.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic significance of tumor laterality in advanced ovarian cancer
- A Case of Recurrent Endometrial Carcinoma at the Vagina
- Analysis of Gynecologic Cancer Registry in Chungnam National University Hospital for 10 years
- Effect of Tumor Grade and FIGO Stage on Preoperative Serum CA 125 Level in Epithelial Ovarian Cancer
- Survival analysis of revised 2013 FIGO staging classification of epithelial ovarian cancer and comparison with previous FIGO staging classification