J Gynecol Oncol.
2013 Jul;24(3):242-248. 10.3802/jgo.2013.24.3.242.
Prognostic factors associated with local recurrence in squamous cell carcinoma of the vulva
- Affiliations
-
- 1Gynecologic Oncology Unit, La Paz University Hospital, Madrid, Spain. ignaciozapardiel@hotmail.com
- KMID: 2288531
- DOI: http://doi.org/10.3802/jgo.2013.24.3.242
Abstract
OBJECTIVE
To analyze the prognostic factors related to the recurrence rate of vulvar cancer.
METHODS
Retrospective study of 87 patients diagnosed of vulvar squamous cell carcinoma diagnosed at a tertiary hospital in Madrid between January 2000 and December 2010.
RESULTS
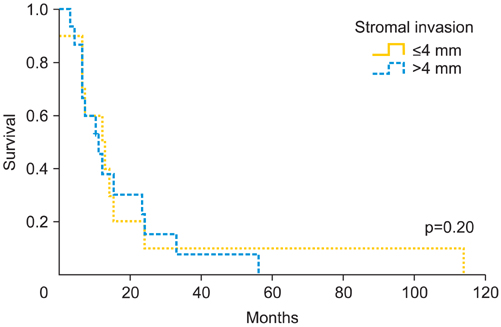
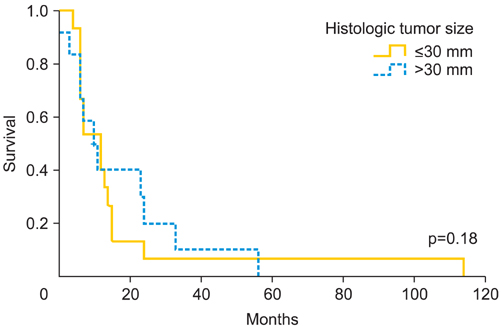
The pathological mean tumor size was 35.1+/-22.8 mm, with stromal invasion of 7.7+/-6.6 mm. The mean free margin after surgery was 16.8+/-10.5 mm. Among all patients, 31 (35.6%) presented local recurrence (mean time 10 months; range, 1 to 114 months) and 7 (8%) had distant metastases (mean time, 5 months; range, 1 to 114 months). We found significant differences in the mean tumor size between patients who presented a relapse and those who did not (37.6+/-21.3 mm vs. 28.9+/-12.1 mm; p=0.05). Patients with free margins equal or less than 8 mm presented a relapse rate of 52.6% vs. 43.5% of those with free margin greater than 8 mm (p=0.50). However, with a cut-off of 15 mm, we observed a local recurrence rate of 55.6% vs. 34.5%, respectively (p=0.09). When the stromal invasion cut-off was >4 mm, local recurrence rate increased up to 52.9% compared to 37.5% when the stromal invasion was < or =4 mm (p=0.20).
CONCLUSION
Tumor size, pathologic margin distance and stromal invasion seem to be the most important predictors of local vulvar recurrence. We consider the cut-off of 35 mm of tumor size, 15 mm tumor-free surgical margin and stromal invasion >4 mm, high risk predictors of local recurrence rate.
MeSH Terms
Figure
Cited by 1 articles
-
Prognostic value of lymph node ratio in surgically treated cases of vulvar cancer: a tertiary care centre experience
Pabashi Poddar, Shilpa Patel, Ruchi Arora, Chetana Parekh, Pariseema Dave, Sangeetha Amin
Obstet Gynecol Sci. 2020;63(2):158-163. doi: 10.5468/ogs.2020.63.2.158.
Reference
-
1. Hacker NF. Vulvar cancer. In : Berek JS, Hacker NF, editors. Practical gynecologic oncology. 4th ed. Philadelphia: Williams & Wilkins;2005. p. 585–602.2. Beller U, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Maisonneuve P, et al. Carcinoma of the vulva: FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006; 95:Suppl 1. S7–S27.3. Joura EA. Epidemiology, diagnosis and treatment of vulvar intraepithelial neoplasia. Curr Opin Obstet Gynecol. 2002; 14:39–43.4. Finan MA, Barre G. Bartholin's gland carcinoma, malignant melanoma and other rare tumours of the vulva. Best Pract Res Clin Obstet Gynaecol. 2003; 17:609–633.5. Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, et al. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study). Am J Obstet Gynecol. 1991; 164:997–1004.6. Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, et al. Prognostic factors for groin node metastasis in squamous cell carcinoma of the vulva (a Gynecologic Oncology Group study). Gynecol Oncol. 1993; 49:279–283.7. de Hullu JA, van der Zee AG. Surgery and radiotherapy in vulvar cancer. Crit Rev Oncol Hematol. 2006; 60:38–58.8. Woelber L, Choschzick M, Eulenburg C, Hager M, Jaenicke F, Gieseking F, et al. Prognostic value of pathological resection margin distance in squamous cell cancer of the vulva. Ann Surg Oncol. 2011; 18:3811–3818.9. Chan JK, Sugiyama V, Pham H, Gu M, Rutgers J, Osann K, et al. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: a multivariate analysis. Gynecol Oncol. 2007; 104:636–641.10. Heaps JM, Fu YS, Montz FJ, Hacker NF, Berek JS. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol. 1990; 38:309–314.11. De Hullu JA, Hollema H, Lolkema S, Boezen M, Boonstra H, Burger MP, et al. Vulvar carcinoma: the price of less radical surgery. Cancer. 2002; 95:2331–2338.12. Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press;2003.13. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009; 105:103–104.14. Podratz KC, Symmonds RE, Taylor WF. Carcinoma of the vulva: analysis of treatment failures. Am J Obstet Gynecol. 1982; 143:340–351.15. Maggino T, Landoni F, Sartori E, Zola P, Gadducci A, Alessi C, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva: a multicenter CTF Study. Cancer. 2000; 89:116–122.16. Palaia I, Bellati F, Calcagno M, Musella A, Perniola G, Panici PB. Invasive vulvar carcinoma and the question of the surgical margin. Int J Gynaecol Obstet. 2011; 114:120–123.17. Groenen SM, Timmers PJ, Burger CW. Recurrence rate in vulvar carcinoma in relation to pathological margin distance. Int J Gynecol Cancer. 2010; 20:869–873.18. Spanish Society of Obstetrics and Gynecologist. Squamous cell invasive vulvar cancer: oncological guidelines. Madrid: SEGO;2010.19. Rouzier R, Morice P, Haie-Meder C, Lhomme C, Avril MF, Duvillard P, et al. Prognostic significance of epithelial disorders adjacent to invasive vulvar carcinomas. Gynecol Oncol. 2001; 81:414–419.20. Tantipalakorn C, Robertson G, Marsden DE, Gebski V, Hacker NF. Outcome and patterns of recurrence for International Federation of Gynecology and Obstetrics (FIGO) stages I and II squamous cell vulvar cancer. Obstet Gynecol. 2009; 113:895–901.21. Woelber L, Mahner S, Voelker K, Eulenburg CZ, Gieseking F, Choschzick M, et al. Clinicopathological prognostic factors andpatterns of recurrence in vulvar cancer. Anticancer Res. 2009; 29:545–552.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Verrucous Carcinoma of Vulva
- A Case of Squamous Cell Carcinoma following Erosive Lichen Planus of the Vulva
- A Case of Vulvar Carcinoma: Squamous Cell Carcinoma with Sarcomatoid Features
- Adenoid Squamous Cell Carcinoma of the Vulva: Report of a case
- Squamous Cell Carcinoma Occurred Concomitantly in the Female Urethra and Vulva