J Clin Neurol.
2006 Mar;2(1):12-28. 10.3988/jcn.2006.2.1.12.
Therapeutic Effects of Caloric Stimulation and Optokinetic Stimulation on Hemispatial Neglect
- Affiliations
-
- 1Department of Neurology, Sungkyunkwan University School of Medicine, Seoul, Korea. dukna@smc.samsung.co.kr
- KMID: 2287699
- DOI: http://doi.org/10.3988/jcn.2006.2.1.12
Abstract
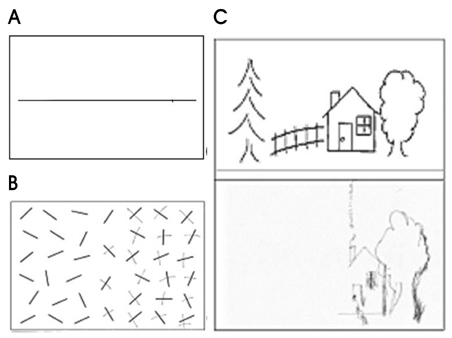
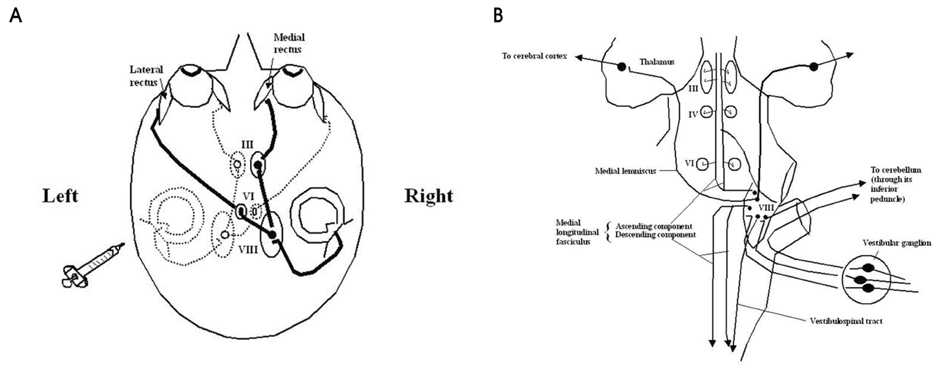
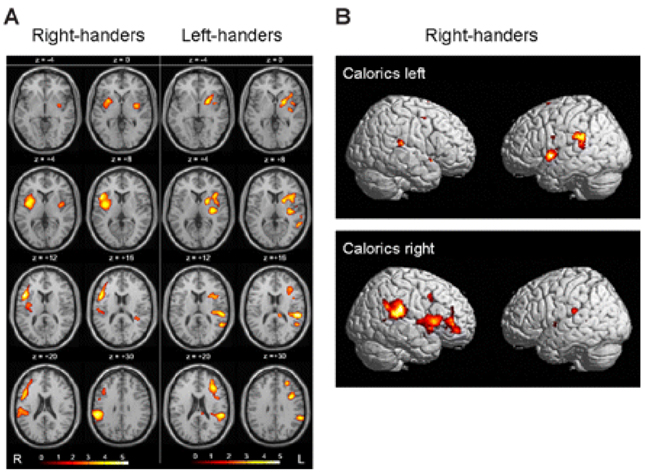
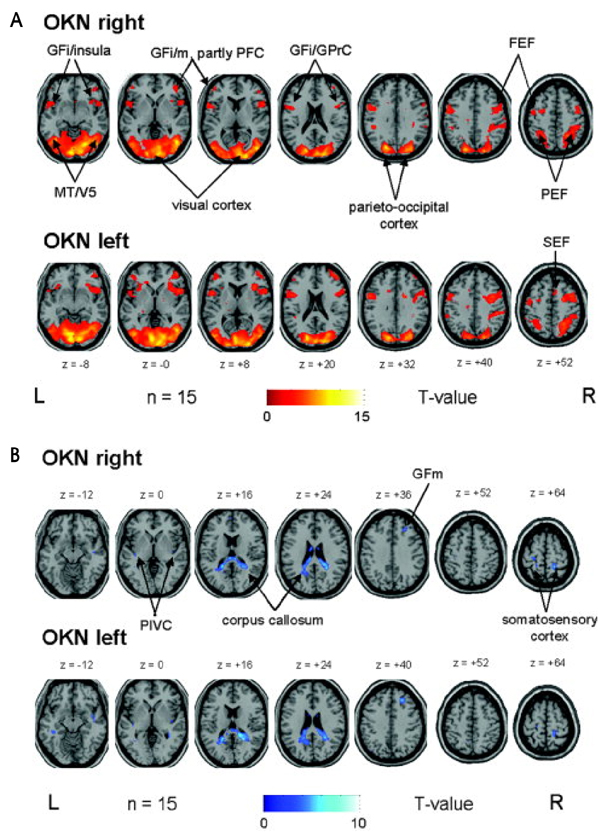
- Hemispatial neglect refers to a cognitive disorder in which patients with unilateral brain injury cannot recognize or respond to stimuli located in the contralesional hemispace. Hemispatial neglect in stroke patients is an important predictor for poor functional outcome. Therefore, there is a need for effective treatment for this condition. A number of interventions for hemispatial neglect have been proposed, although an approach resulting in persistent improvement is not available. Of these interventions, our review is focused on caloric stimulation and optokinetic stimulation. These lateralized or direction-specific stimulations of peripheral sensory systems can temporarily improve hemispatial neglect. According to recent functional MRI and PET studies, this improvement might result from the partial (re)activation of a distributed, multisensory vestibular network in the lesioned hemisphere, which is a part of a system that codes ego-centered space. However, much remain unknown regarding exact signal timing and directional selectivity of the network.
Keyword
Figure
Reference
-
1. Heilman K.M., Watson R.T., Valenstein E. Heilman K.M., Valenstein E, editors. Neglect and related disorders. Clinical Neuropsychology. 2003. 4th ed. New York: Oxford University Press;296–346.2. Gainotti G, Messerli P, Tissot R. Qualitative analysis of unilateral spatial neglect in relation to laterality of cerebral lesions. J Neurol Neurosurg Psychiatry. 1972. 35:545–550.
Article3. Hier DB, Mondlock J, Caplan LR. Recovery of behavioral abnormalities after right hemisphere stroke. Neurology. 1983. 33:345–350.
Article4. Stone SP, Wilson B, Wroot A, Halligan PW, Lange LS, Marshall JC, Greenwood RJ, et al. The assessment of visuo-spatial neglect after acute stroke. J Neurol Neurosurg Psychiatry. 1991. 54:345–350.
Article5. Albert ML. A simple test of visual neglect. Neurology. 1973. 23:658–664.
Article6. Gauthier L, Dehaut F, Joanette Y. The bells test: a quantitative and qualitative test for visual neglect. Int Clin Neuropsychol. 1989. 11:49–54.7. Ogden JA. Anterior-posterior interhemispheric differences in the loci of lesions producing visual hemineglect. Brain and Cognition. 1985. 4:59–75.
Article8. Halligan PW, Marshall JC, Wade DT. Visuospatial neglect: underlying factors and test sensitivity. Lancet. 1989. 2:908–911.
Article9. Denes G, Semenza C, Stoppa E, Lis A. Unilateral spatial neglect and recovery from hemiplegia: a follow-up study. Brain. 1982. 105:543–552.
Article10. Katz N, Hartman-Maeir A, Ring H, Soroker N. Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Arch Phys Med Rehabil. 1999. 80:379–384.
Article11. Karnath HO, Zihl J. Brandt T, Caplan LR, Dichgans J, Diener HC, Kennard C, editors. Disorders of spatial orientation. Neurological disorders: course and treatment. 2003. San Diego, CA: Academic Press;277–286.
Article12. Lawson IR. Visual-spatial neglect in lesions of the right cerebral hemisphere. A study in recovery. Neurology. 1962. 12:23–33.
Article13. Pizzamiglio L, Antonucci G, Judica A, Montenero P, Razzano C, Zoccolotti P. Cognitive rehabilitation of the hemineglect disorder in chronic patients with unilateral right brain damage. J Clin Exp Neuropsychol. 1992. 14:901–923.
Article14. Robertson IH, North N. Spatio-motor cueing in unilateral left neglect: the role of hemispace, hand and motor activation. Neuropsychologia. 1992. 30:553–563.
Article15. Liepert J. Transcranial magnetic stimulation in neurorehabilitation. Acta Neurochir Suppl. 2005. 93:71–74.
Article16. Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. 1998. 395:166–169.
Article17. Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966. 153:1544–1547.
Article18. Arai T, Ohi H, Sasaki H, Nobuto H, Tanaka K. Hemispatial sunglasses: effect on unilateral spatial neglect. Arch Phys Med Rehabil. 1997. 78:230–232.
Article19. Beis JM, Andre JM, Baumgarten A, Challier B. Eye patching in unilateral spatial neglect: efficacy of two methods. Arch Phys Med Rehabil. 1999. 80:71–76.
Article20. Na DL, Adair JC, Williamson DJ, Schwartz RL, Haws B, Heilman KM. Dissociation of sensory-attentional from motor-intentional neglect. J Neurol Neurosurg Psychiatry. 1998. 64:331–338.
Article21. Bankiewicz KS, Oldfield EH, Plunkett RJ, Schuette WH, Cogan DG, Hogan N, et al. Apparent unilateral visual neglect in MPTP-hemiparkinsonian monkeys is due to delayed initiation of motion. Brain Res. 1991. 541:98–102.
Article22. Fleet WS, Valenstein E, Watson RT, Heilman KM. Dopamine agonist therapy for neglect in humans. Neurology. 1987. 37:1765–1770.
Article23. Geminiani G, Bottini G, Sterzi R. Dopaminergic stimulation in unilateral neglect. J Neurol Neurosurg Psychiatry. 1998. 65:344–347.
Article24. Abzug C, Maeda M, Peterson BW, Wilson VJ. Cervical branching of lumbar vestibulo-spinal axons. J Physiol. 1974. 243:499–522.
Article25. Iwamoto Y, Perlmutter SI, Baker JF, Peterson BW. Spatial coordination by descending vestibular signals. 2. Response properties of medial and lateral vestibulospinal tract neurons in alert and decerebrate cats. Exp Brain Res. 1996. 108:85–100.26. Nathan PW, Smith M, Deacon P. Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain. 1996. 119:1809–1833.
Article27. Nishiike S, Guldin WO, Baurle J. Corticofugal connections between the cerebral cortex and vestibular nuclei in rat. J Comp Neurol. 2000. 420:363–372.
Article28. Akbarian S, Grüsser O-J, Guldin WO. Corticofugal connections between the cerebral cortex and brainstem vestibular nuclei in the macaque monkey. J Comp Neurol. 1994. 339:421–437.
Article29. Fredrickson JM, Figge U, Scheid P, Kornhuber HH. Vestibular nerve projection to the cerebral cortex of the rhesus monkey. Exp Brain Res. 1966. 2:318–327.
Article30. Schwarz DWF, Fredrickson JM. Rhesus monkey vestibular cortex: a bimodal primary projection field. Science. 1971. 172:280–281.
Article31. Ödkvist LM, Schwarz DWF, Fredrickson JM, Hassler R. Projection of the vestibular nerve to the area 3a arm field in the squirrel monkey (Saimiri sciureus). Exp Brain Res. 1974. 21:97–105.
Article32. Büttner U, Buettner UW. Parietal cortex (2v) neuronal activity in the alert monkey during natural vestibular and OKS. Brain Res. 1978. 153:392–397.
Article33. Faugier-Grimaud S, Ventre J. Anatomic connections of inferior parietal cortex (Area 7) with subcortical structures related to vestibulo-ocular function in a monkey (Macaca fascicularis). J Comp Neurol. 1989. 280:1–14.
Article34. Grüsser OJ, Pause M, Schreiter U. Localization and responses of neurons in the parieto-insular cortex of awake monkeys (Macaca fascicularis). J Physiol (Lond). 1990. 430:537–557.
Article35. Grüsser OJ, Pause M, Schreiter U. Vestibular neurones in the parieto-insular cortex of monkeys (Macaca fascicularis): visual and neck receptor responses. J Physiol. 1990. 430:559–583.
Article36. Guldin WO, Grüsser OJ. Collard M, Jeannerod M, Christen Y, editors. The anatomy of the vestibular cortices of primates. Le cortex vestibulaire. 1996. Editions IRVINN. Paris: Ipsen;17–26.37. Bremmer F, Klam F, Duhamel J-R, Hamed SB, Graf W. Visual-vestibular interactive responses in the macaque ventral intraparietal area (VIP). Eur J Neurosci. 2002. 16:1569–1586.
Article38. Klam F, Graf W. Vestibular signals of posterior parietal cortex neurons during active and passive head movements in macaque monkeys. Ann N Y Acad Sci. 2003. 1004:271–282.
Article39. Klam F, Graf W. Vestibular response kinematics in posterior parietal cortex neurons of macaque monkeys. Eur J Neurosci. 2003. 18:995–1010.
Article40. Ebata S, Sugiuchi Y, Izawa Y, Shinomiya K, Shinoda Y. Vestibular projection to the periarcuate cortex in the monkey. Neurosci Res. 2004. 49:55–68.
Article41. Schlack A, Sterbing-D'Angelo SJ, Hartung K, Hoffmann KP, Bremmer F. Multisensory space representations in the macaque ventral intraparietal area. J Neurosci. 2005. 25:4616–4625.
Article42. Bottini G, Sterzi R, Paulesu E, Vallar G, Cappa SF, Erminio F, et al. Identification of the central vestibular projections in man: a positron emission tomography activation study. Exp Brain Res. 1994. 99:164–169.
Article43. Bucher SF, Dieterich M, Wiesmann M, Weiss A, Zink R, Yousry T, et al. Cerebral functional MRI of vestibular, auditory, and nociceptive areas during galvanic stimulation. Ann Neurol. 1998. 44:120–125.
Article44. Lobel E, Kleine JF, Le Bihan D, Leroy-Willig A, Berthoz A. Functional MRI of galvanic vestibular stimulation. J Neurophysiol. 1998. 80:2699–2709.
Article45. Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol. 2001. 85:886–899.
Article46. Bremmer F, Schlack A, Duhamel J-R, Graf W, Fink GR. Space coding in primate posterior parietal cortex. Neuroimage. 2001. 14:S46–S51.
Article47. Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogawa T, et al. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2001. 12:441–449.
Article48. Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, et al. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002. 17:1384–1393.
Article49. Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Brandt T, et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cerebral Cortex. 2003. 13:994–1007.
Article50. Emri M, Kisely M, Lengyel Z, Balkay L, Marian T, Miko L, et al. Cortical projection of peripheral vestibular signaling. J Neurophysiol. 2003. 89:2639–2646.
Article51. Stephan T, Deutschländer A, Nolte A, Schneider E, Wiesmann M, Brandt T, et al. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage. 2005. 26:721–732.
Article52. Wenzel R, Bartenstein P, Dieterich M, Danek A, Weindl A, Minoshima S, et al. Deactivation of human visual cortex during involuntary ocular oscillations. A PET activation study. Brain. 1996. 119:101–110.
Article53. Dieterich M, Bartenstein P, Spiegel S, Bense S, Schwaiger M, Brandt T. Thalamic infarctions cause side-specific suppression of vestibular cortex activations. Brain. 2005. 128:2052–2067.
Article54. Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988. 240:740–749.
Article55. Tusa RJ, Ungerleider L. Fiber pathways of cortical areas mediating smooth pursuit eye movements in monkeys. Ann Neurol. 1988. 23:174–183.
Article56. Ffytche DH, Guy CN, Zeki S. The parallel visual motion inputs into areas V1 and V5 of human cerebral cortex. Brain. 1995. 118:1375–1394.
Article57. Rodman HR, Gross CG, Albright TD. Afferent basis of visual response properties in area MT of the macaque. I. Effect of striate cortex removal. J Neurosci. 1989. 9:2033–2050.
Article58. Desimone R, Ungerleider LG. Multiple visual areas in the causal superior temporal sulcus of the macaque. J Comp Neurol. 1986. 248:164–189.
Article59. Komatsu H, Wurtz RH. Relation of cerebral areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol. 1988. 60:580–603.
Article60. Maunsell JHR, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol. 1983. 49:1127–1147.
Article61. Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal area (MT). J Neurosci. 1988. 8:2201–2211.
Article62. Dürsteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol. 1988. 60:940–965.
Article63. Tootell RB, Taylor JB. Anatomical evidence for MT and additional cortical visual areas in humans. Cerebral Cortex. 1995. 5:39–55.
Article64. Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSI. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1997. 11:641–649.
Article65. Barton JJS, Sharpe JA, Raymond JE. Retinotopic and directional defects in motion discrimination in humans with cerebral lesions. Ann Neurol. 1995. 37:665–675.
Article66. Barton JJS, Sharpe JA, Raymond JE. Directional defects in pursuit and motion perception in humans with unilateral cerebral lesions. Brain. 1996. 119:1535–1550.
Article67. Zihl J, Von Crammon D, Mai N. Selective disturbance of movement vision after bilateral nrain damage. Brain. 1983. 106:313–340.
Article68. Morrow MJ, Sharpe JA. Cerebral hemispheric localization of smooth pursuit asymmetry. Neurology. 1990. 40:284–292.
Article69. Thurston SE, Leigh RJ, Crawford T, Thompson A, Kennard C. Two distinct deficits of visual tracking caused by unilateral lesions of cerebral cortex in man. Ann Neurol. 1988. 23:266–273.
Article70. Felleman DJ, Van Essen DC. Distributed hierarchial processing in the primate cerebral cortex. Cereb Cortex. 1991. 1:1–47.71. Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation od retinal from extraretinal inputs. J Neurophysiol. 1988. 60:604–620.
Article72. Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large field stimuli. J Neurophysiol. 1991. 65:1329–1345.
Article73. Graziano MS, Anderson RA, Snowden RJ. Tuning of MST neurons to spiral motions. J Neurosci. 1994. 14:54–57.
Article74. Barton JJS, Simpson T, Kiriakopoulos E, Stewart C, Crawley A, Gauthrie B, et al. Functional MRI of lateral occipitotemporal cortex during pursuit and motion perception. Ann Neurol. 1996. 40:387–398.
Article75. Scalaidhe SP, Albright TD, Rodman HR, Gross CG. Effect of superior temporal polysensory area lesions on eye movements in the macaque monkey. J Neurophysiol. 1995. 73:1–19.
Article76. Brandt SA, Dale AM, Wenzel R, Culham JC, Mendola JD, Tootel RBH. Sensory, motor and attentional components of eye movement induced cortex activation revealed by fMRI. Soc Neurosci Abstr. 1997. 23:2223.77. Glickstein M, Cohen JL, Dixon B, Gibson A, Hollins M, Labossiere E, Robinson F, et al. Corticopontine visual projections in macaque monkeys. J Comp Neurol. 1980. 190:209–229.
Article78. May RJ, Keller EL, Suzuki DA. Smooth-pursuit eye movement deficits with chemical lesions in the dorsolateral pontine nucleus of the monkey. J Neurophysiol. 1988. 59:952–977.
Article79. Mustari MJ, Fuchs AF, Wallman J. Response properties of dorsolateral pontine units during smooth pursuit in the rhesus macaque. J Neurophysiol. 1988. 60:664–686.
Article80. Glickstein M, Gerrits N, Kralj-hans I, Mercier B, Stein J, Voogd J. Visual pontocerebellar projections in the macaque. J Comp Neurol. 1994. 349:51–72.
Article81. Brodal P. Further observations on the cerebellar projections from the pontine nuclei and the nucleus reticularis tegmenti pontis in the rhesus monkey. J Comp Neurol. 1982. 204:44–55.
Article82. Bucher SF, Dieterich M, Seelos KC, Brandt T. Sensorimotor cerebral activation during optokinetic nystagmus. A functional MRI study. Neurology. 1997. 49:1370–1377.
Article83. Dieterich M, Bucher SF, Seelos KC, Brandt T. Horizontal or vertical OKS activates visual motion-sensitive, ocular motor and vestibular cortex areas with right hemispheric dominance. An fMRI study. Brain. 1998. 121:1479–1495.
Article84. Galati G, Pappata S, Pantano P, Lenzi GL, Samson Y, Pizzamiglio L. Cortical control of optokinetic nystagmus in humans: a positron emission tomography study. Exp Brain Res. 1999. 126:149–159.
Article85. Rosano C, Krisky CM, Welling JS, Eddy WF, Luna B, Thulborn KR, Sweeny JA, et al. Pursuit and saccadic eye movement subregions in human frontal eye fields: a high-resolution fMRI investigation. Cereb Cortex. 2002. 12:107–115.
Article86. Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996. 34:475–483.
Article87. Pierrot-Deseilligny C, Milea D, Müri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004. 17:17–25.
Article88. Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, et al. A large-scale distributed network for covert spatial attention. Further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999. 122:1093–1106.89. Gitelman DR, Parrish TB, Friston KJ, Mesulam MM. Functional anatomy of visual search: regional segregations within the frontal eye fields and effective connectivity of the superior colliculus. Neuroimage. 2002. 15:970–982.
Article90. Weber B, Schwarz U, Kneifel S, Treyer V, Buck A. Hierarchical visual processing is dependent on the oculomotor system. Neuroreport. 2000. 11:241–247.
Article91. Bense S, Janusch B, Schlindwein P, Bauermann T, Vucurevic G, Brandt T, et al. Direction-dependent visual cortex activation during horizontal OKS (fMRI study). Hum Brain Mapp. 2005. 08. 03. [Epub ahead of print].92. Gottlieb JP, MacAvoy MG, Bruce CJ. Neuronal responses related to smooth pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J Neurophysiol. 1994. 72:1634–1653.
Article93. Hoffmann K-P, Bremmer F, Thiele A, Distler C. Directional asymmetry of neurons in cortical areas MT and MST projecting to the NOT-DTN in macaques. J Neurophysiol. 2002. 87:2113–2123.
Article94. Huk AC, Ress D, Heeger DJ. Neuronal basis of the motion aftereffect reconsidered. Neuron. 2001. 32:161–172.
Article95. Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual-vestibular interaction: visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain. 1998. 121:1749–1758.
Article96. Abderhalden E. Lehrbuch der Physiologie in Vorlesungen. 1926. Bd. 3. Berlin: Urban & Schwarzenberg.97. Jung R. Bergmann Gv, Frey W, Schwiegk H, editors. Neurophysiologische Untersuchungsmethoden. Handbuch der Inneren Medizin. 1953. Bd. V. Berlin: Springer;1206–1420.
Article98. Karnath H-O, Himmelbach M, Perenin M-T. Neglect-like behaviour in healthy subjects: dissociation of space exploration and goal-directed pointing following vestibular stimulation. Exp Brain Res. 2003. 153:231–238.99. Rubens AB. Caloric stimulation and unilateral visual neglect. Neurology. 1985. 35:1019–1024.
Article100. Cappa SF, Sterzi R, Vallar G, Bisiach E. Remission of hemineglect and anosognosia during vestibular stimulation. Neuropsychologia. 1987. 25:775–782.
Article101. Geminiani G, Bottini G. Mental representation and temporary recovery from unilateral neglect after vestibular stimulation. J Neurol Neurosurg Psychiatry. 1992. 55:332–333.
Article102. Rode G, Charles N, Perenin MT, Vighetto A, Trillet M, Aimard G. Partial remission of hemiplegia and somatoparaphrenia through vestibular stimulation in a case of unilateral neglect. Cortex. 1992. 28:203–208.
Article103. Adair JC, Na DL, Schwartz RL, Heilman KM. Caloric stimulation in neglect: evaluation of response as a function of neglect type. J Int Neuropsychol Soc. 2003. 9:983–988.
Article104. Storrie-Baker HJ, Segalowitz SJ, Black SE, McLean JA, Sullivan N. Improvement of hemispatial neglect with cold-water calorics: an electrophysiological test of the arousal hypothesis of neglect. J Int Neuropsychol Soc. 1997. 3:394–402.
Article105. Karnath HO. Subjective body orientation in neglect and the interactive contribution of neck muscle proprioception and vestibular stimulation. Brain. 1994. 117:1001–1012.
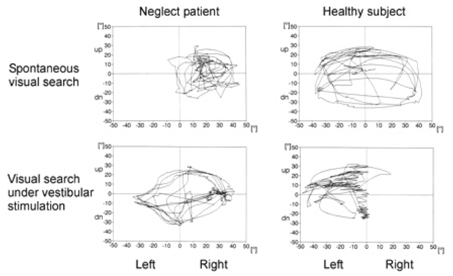
Article106. Karnath HO, Dieterich M. Spatial neglect--a vestibular disorder? Brain. 2006. 129:293–305.
Article107. Mattingley JB, Bradshaw JL, Bradshaw JA. Horizontal visual motion modulates focal attention in left unilateral spatial neglect. J Neurol Neurosurg Psychiatry. 1994. 57:1228–1235.
Article108. Bisiach E, Pizzamiglio L, Nico D, Antonucci G. Beyond unilateral neglect. Brain. 1996. 119:851–857.
Article109. Pizzamiglio L, Frasca R, Guariglia C, Incoccia C, Antonucci G. Effect of OKS in patients with visual neglect. Cortex. 1990. 26:535–540.110. Zoccolotti P, Guariglia C, Pizzamiglio L, Judica A, Razzano C, Pantano P. Good recovery in visual scanning in a patient with persistent anosognosia. Int J Neurosci. 1992. 63:93–104.
Article111. Vallar G, Antonucci G, Guariglia C, Pizzamiglio L. Deficits of position sense, unilateral neglect and OKS. Neuropsychologia. 1993. 31:1191–1200.
Article112. Vallar G, Guariglia C, Magnotti L, Pizzamiglio L. OKS affects both vertical and horizontal deficits of position sense in unilateral neglect. Cortex. 1995. 31:669–683.
Article113. Karnath HO. OKS influences the disturbed perception of body orientation in spatial neglect. J Neurol Neurosurg Psychiatry. 1996. 60:217–220.
Article114. Vallar G, Guariglia C, Nico D, Pizzamiglio L. Motor deficits and OKS in patients with left hemineglect. Neurology. 1997. 49:1364–1370.
Article115. Vallar G, Guariglia C, Magnotti L, Pizzamiglio L. Dissociation between position sense and visual-spatial components of hemineglect through a specific rehabilitation treatment. J Clin Exp Neuropsychol. 1997. 19:763–771.
Article116. Kerkhoff G, Schindler I, Keller I, Marquardt C. Visual background motion reduces size distortion in spatial neglect. Neuroreport. 1999. 10:319–323.
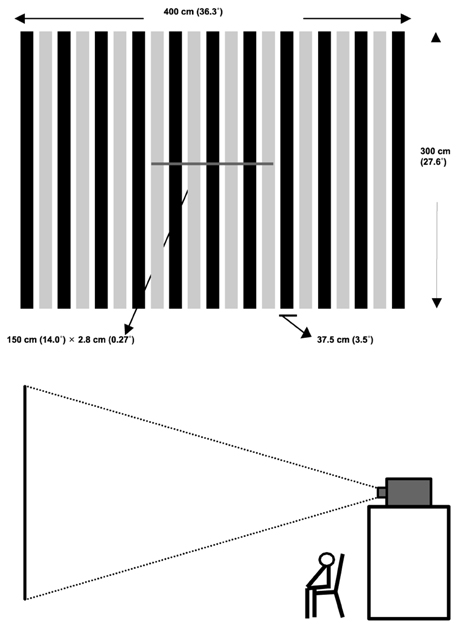
Article117. Pizzamiglio L, Fasotti L, Jehkonen M, Antonucci G, Magnotti L, Boelen D, et al. The use of OKS in rehabilitation of the hemineglect disorder. Cortex. 2004. 40:441–450.
Article118. Na DL, Son Y, Kim CH, Lee BH, Shon YM, Lee KJ, et al. Effect of background motion on line bisection performance in normal subjects. Cortex. 2002. 38:787–796.
Article119. Jeong Y, Lee BH, Ahn HJ, Park KC, Heilman KM, Na DL. Attentional bias induced by viewing actual or illusory movements. Neurology. 2004. 62:1333–1337.
Article120. Choi KM, Ku BD, Jeong Y, Lee BH, Ahn HJ, Kang SJ, et al. The influence of illusory motion on line bisection performance in normal subjects. J Int Neuropsychol Soc. 2005. 11(7):881–888.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Number Processing Error as a Clinical Manifestation of Hemispatial Neglect Following Hypoxic Brain Injury:a Case Report
- Influence of Hemispatial Neglect on Trunk Control in Stroke Patients
- Efficacy of Unilateral and Bilateral Parietal Transcranial Direct Current Stimulation on Right Hemispheric Stroke Patients With Neglect Symptoms: A Proof-of-Principle Study
- Correlation of Hemispatial Neglect with White Matter Tract Integrity: A DTI Study
- Effect of Combined Therapy of Robot and Low-Frequency Repetitive Transcranial Magnetic Stimulation on Hemispatial Neglect in Stroke Patients