J Clin Neurol.
2008 Mar;4(1):1-16. 10.3988/jcn.2008.4.1.1.
Role of Neuroimaging in the Presurgical Evaluation of Epilepsy
- Affiliations
-
- 1Epilepsy Center-S51, Neurological Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195 USA. wehnert@ccf.org
- KMID: 2287679
- DOI: http://doi.org/10.3988/jcn.2008.4.1.1
Abstract
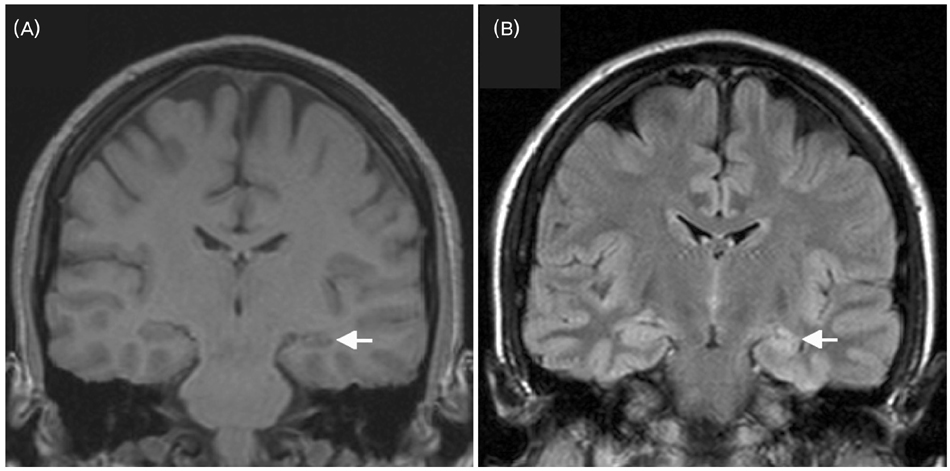
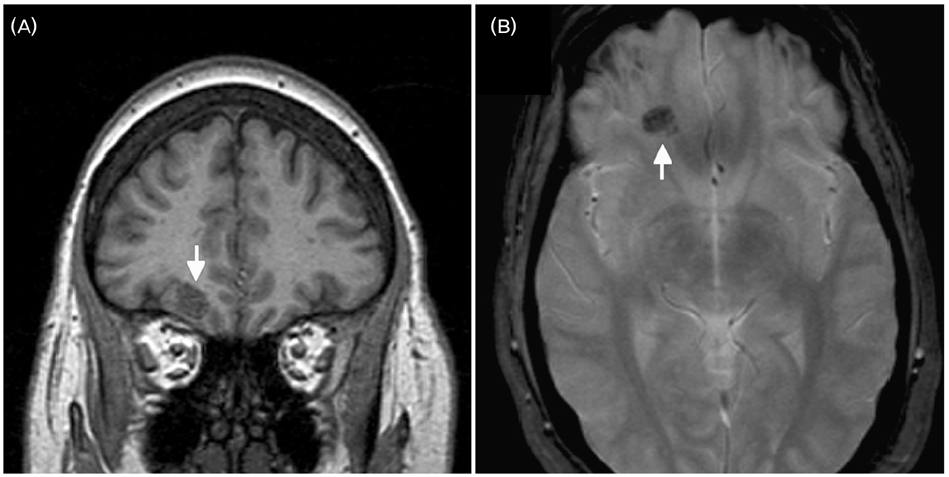
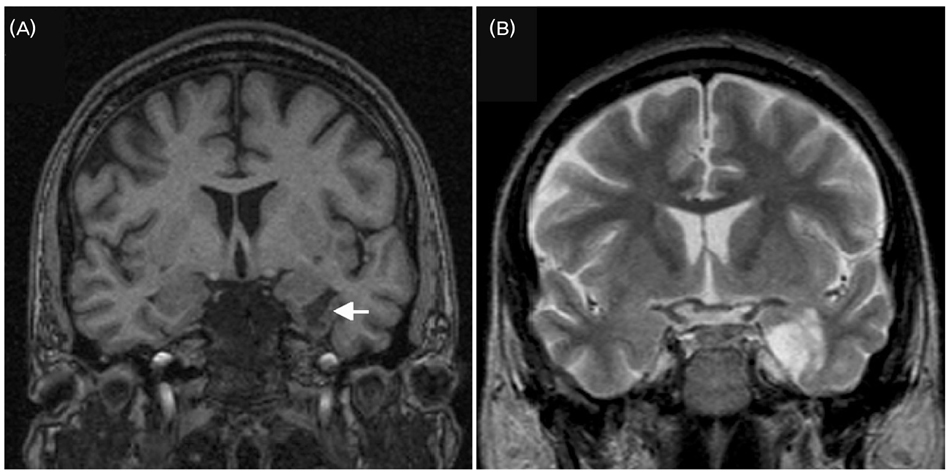
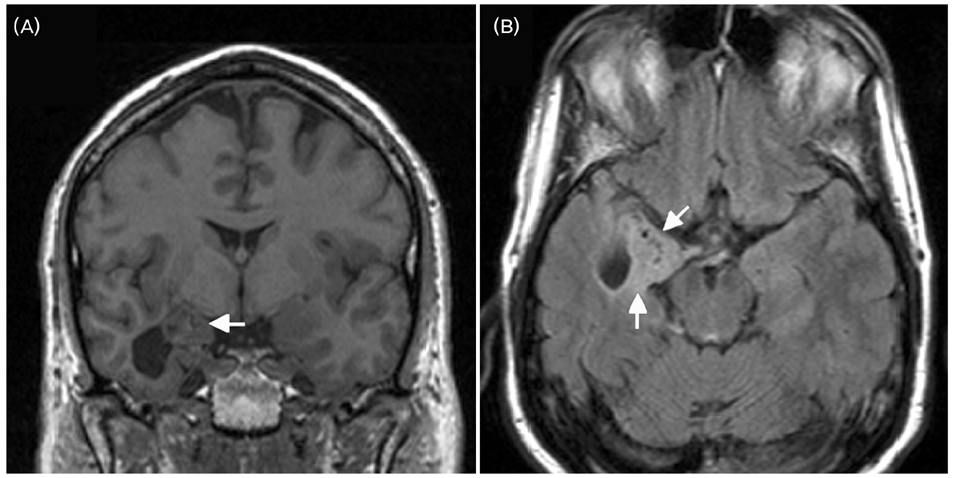
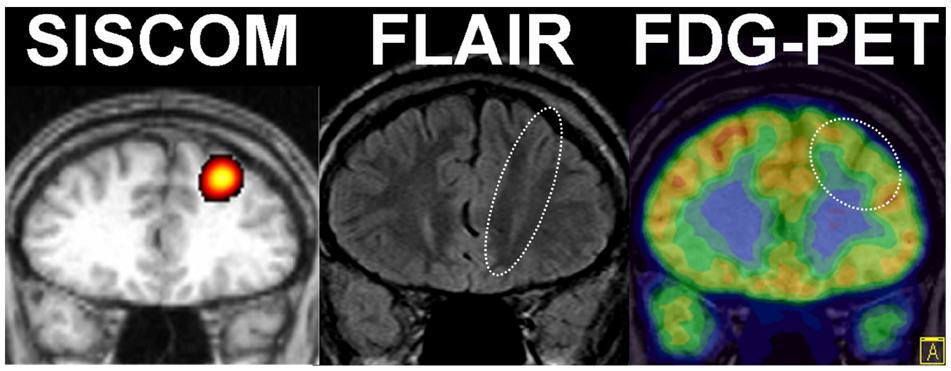
- A significant minority of patients with focal epilepsy are candidates for resective epilepsy surgery. Structural and functional neuroimaging plays an important role in the presurgical evaluation of theses patients. The most frequent etiologies of pharmacoresistant epilepsy in the adult population are mesial temporal sclerosis, malformations of cortical development, cavernous angiomas, and low-grade neoplasms. High-resolution multiplanar magnetic resonance imaging (MRI) with sequences providing T1 and T2 contrast is the initial imaging study of choice to detect these epileptogenic lesions. The epilepsy MRI protocol can be individually tailored when considering the patient's clinical and electrophysiological data. Metabolic imaging techniques such as positron emission tomography (PET) and single photon emission tomography (SPECT) visualize metabolic alterations of the brain in the ictal and interictal states. These techniques may have localizing value in patients with a normal MRI scan. Functional MRI is helpful in non-invasively identifying areas of eloquent cortex.
MeSH Terms
Figure
Cited by 2 articles
-
Neurocutaneous Melanosis Presenting as Chronic Partial Epilepsy
Byoung Seok Ye, Yang-Je Cho, Sang Hyun Jang, Byung In Lee, Kyoung Heo, Hyun Ho Jung, Jin Woo Chang, Se Hoon Kim
J Clin Neurol. 2008;4(3):134-137. doi: 10.3988/jcn.2008.4.3.134.Gray Matter Concentration Abnormality in Brains of Narcolepsy Patients
Eun Yeon Joo, Woo Suk Tae, Sung Tae Kim, Seung Bong Hong
Korean J Radiol. 2009;10(6):552-558. doi: 10.3348/kjr.2009.10.6.552.
Reference
-
1. Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001. 124:1683–1700.
Article2. Engel J Jr. Surgery for seizures. N Engl J Med. 1996. 334:647–652.
Article3. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001. 345:311–318.
Article4. Raybaud C, Shroff M, Rutka JT, Chuang SH. Imaging surgical epilepsy in children. Childs Nerv Syst. 2006. 22:786–809.
Article5. Koepp MJ, Woermann FG. Imaging structure and function in refractory focal epilepsy. Lancet Neurol. 2005. 4:42–53.
Article6. Cardoso TA, Coan AC, Kobayashi E, Guerreiro CA, Li LM, Cendes F. Hippocampal abnormalities and seizure recurrence after antiepileptic drug withdrawal. Neurology. 2006. 67:134–136.
Article7. Spooner CG, Berkovic SF, Mitchell LA, Wrennall JA, Harvey AS. New-onset temporal lobe epilepsy in children: Lesion on MRI predicts poor seizure outcome. Neurology. 2006. 67:2147–2153.
Article8. Berkovic SF, McIntosh AM, Kalnins RM, Jackson GD, Fabinyi GC, Brazenor GA, et al. Preoperative MRI predicts outcome of temporal lobectomy: An actuarial analysis. Neurology. 1995. 45:1358–1363.
Article9. Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Luders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007. 130:574–584.
Article10. Beaumont A, Whittle IR. The pathogenesis of tumour associated epilepsy. Acta Neurochir (Wien). 2000. 142:1–15.
Article11. Krumholz A, Wiebe S, Gronseth G, Shinnar S, Levisohn P, Ting T, et al. Practice parameter: Evaluating an apparent unprovoked first seizure in adults (an evidence-based review): Report of the quality standards subcommittee of the american academy of neurology and the american epilepsy society. Neurology. 2007. 69:1996–2007.
Article12. ACEP Clinical Policies Committee. Clinical Policies Subcommittee on Seizures. Clinical policy: Critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med. 2004. 43:605–625.13. Recommendations for neuroimaging of patients with epilepsy. commission on neuroimaging of the international league against epilepsy. Epilepsia. 1997. 38:1255–1256.14. Guidelines for neuroimaging evaluation of patients with uncontrolled epilepsy considered for surgery. Commission on Neuroimaging of the International League Against Epilepsy. Epilepsia. 1998. 39:1375–1376.15. Von Oertzen J, Urbach H, Jungbluth S, Kurthen M, Reuber M, Fernandez G, et al. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry. 2002. 73:643–647.
Article16. Knake S, Triantafyllou C, Wald LL, Wiggins G, Kirk GP, Larsson PG, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: A prospective study. Neurology. 2005. 65:1026–1031.
Article17. Bronen RA, Fulbright RK, Kim JH, Spencer SS, Spencer DD. A systematic approach for interpreting MR images of the seizure patient. AJR Am J Roentgenol. 1997. 169:241–247.
Article18. Jackson GD, Berkovic SF, Tress BM, Kalnins RM, Fabinyi GC, Bladin PF. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990. 40:1869–1875.
Article19. Oppenheim C, Dormont D, Biondi A, Lehericy S, Hasboun D, Clemenceau S, et al. Loss of digitations of the hippocampal head on high-resolution fast spinecho MR: A sign of mesial temporal sclerosis. AJNR Am J Neuroradiol. 1998. 19:457–463.20. Diehl B. Rosenow F, Luders H, editors. CT scan and MRI in the definition of the epileptogenic lesion. Presurgical assessment of the epilepsies with clinical neurophysiology and functional imaging. Handbook of Clinical Neurophysiology. 2004. Elsevier;199–216.
Article21. Liu RS, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JW, et al. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. NeuroImage. 2003. 20:22–33.
Article22. Liu RS, Lemieux L, Bell GS, Sisodiya SM, Bartlett PA, Shorvon SD, et al. Cerebral damage in epilepsy: A population-based longitudinal quantitative MRI study. Epilepsia. 2005. 46:1482–1494.
Article23. Duncan JS. Imaging and epilepsy. Brain. 1997. 120(Pt 2):339–377.
Article24. von Oertzen J, Urbach H, Blumcke I, Reuber M, Traber F, Peveling T, et al. Time-efficient T2 relaxometry of the entire hippocampus is feasible in temporal lobe epilepsy. Neurology. 2002. 58:257–264.
Article25. Widdess-Walsh P, Diehl B, Najm I. Neuroimaging of focal cortical dysplasia. J Neuroimaging. 2006. 16:185–196.
Article26. Diehl B, Symms MR, Boulby PA, Salmenpera T, Wheeler-Kingshott CA, Barker GJ, et al. Postictal diffusion tensor imaging. Epilepsy Res. 2005. 65:137–146.
Article27. Thivard L, Lehericy S, Krainik A, Adam C, Dormont D, Chiras J, et al. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2005. 28:682–690.
Article28. Wehner T, Lapresto E, Tkach J, Liu P, Bingaman W, Prayson RA, et al. The value of interictal diffusionweighted imaging in lateralizing temporal lobe epilepsy. Neurology. 2007. 68:122–127.
Article29. Woo CL, Chuang SH, Becker LE, Jay V, Otsubo H, Rutka JT, et al. Radiologic-pathologic correlation in focal cortical dysplasia and hemimegalencephaly in 18 children. Pediatr Neurol. 2001. 25:295–303.
Article30. Salamon N, Andres M, Chute DJ, Nguyen ST, Chang JW, Huynh MN, et al. Contralateral hemimicrencephaly and clinical-pathological correlations in children with hemimegalencephaly. Brain. 2006. 129:352–365.
Article31. Guerrini R, Sicca F, Parmeggiani L. Epilepsy and malformations of the cerebral cortex. Epileptic Disord. 2003. 5:Suppl 2. S9–S26.32. Sisodiya SM. Surgery for malformations of cortical development causing epilepsy. Brain. 2000. 123:1075–1091.
Article33. Janszky J, Ebner A, Kruse B, Mertens M, Jokeit H, Seitz RJ, et al. Functional organization of the brain with malformations of cortical development. Ann Neurol. 2003. 53:759–767.
Article34. Tassi L, Colombo N, Cossu M, Mai R, Francione S, Lo Russo G, et al. Electroclinical, MRI and neuropathological study of 10 patients with nodular heterotopia, with surgical outcomes. Brain. 2005. 128:321–337.
Article35. Chapman K, Wyllie E, Najm I, Ruggieri P, Bingaman W, Luders J, et al. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry. 2005. 76:710–713.
Article36. Ruggieri PM, Najm I, Bronen R, Campos M, Cendes F, Duncan JS, et al. Neuroimaging of the cortical dysplasias. Neurology. 2004. 62:S27–S29.
Article37. Colliot O, Mansi T, Bernasconi N, Naessens V, Klironomos D, Bernasconi A. Segmentation of focal cortical dysplasia lesions on MRI using level set evolution. Neuroimage. 2006. 32:1621–1630.
Article38. Colliot O, Antel SB, Naessens VB, Bernasconi N, Bernasconi A. In vivo profiling of focal cortical dysplasia on high-resolution MRI with computational models. Epilepsia. 2006. 47:134–142.
Article39. Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, et al. Terminology and classification of the cortical dysplasias. Neurology. 2004. 62:S2–S8.
Article40. Colombo N, Tassi L, Galli C, Citterio A, Lo Russo G, Scialfa G, et al. Focal corticaldysplasias: MR imaging, histopathologic, and clinical correlations in surgically treated patients with epilepsy. AJNR Am J Neuroradiol. 2003. 24:724–733.41. Widdess-Walsh P, Kellinghaus C, Jeha L, Kotagal P, Prayson R, Bingaman W, et al. Electro-clinical and imaging characteristics of focal cortical dysplasia: Correlation with pathological subtypes. Epilepsy Res. 2005. 67:25–33.
Article42. Marusic P, Najm IM, Ying Z, Prayson R, Rona S, Nair D, et al. Focal cortical dysplasias in eloquent cortex: Functional characteristics and correlation with MRI and histopathologic changes. Epilepsia. 2002. 43:27–32.
Article43. Bernasconi A. Advanced MRI analysis methods for detection of focal cortical dysplasia. Epileptic Disord. 2003. 5:Suppl 2. S81–S84.44. Osborn AG, et al. Diagnostic imaging: Brain. 2004. Salt Lake City: Amirsys.45. Baumann CR, Schuknecht B, Lo Russo G, Cossu M, Citterio A, Andermann F, et al. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia. 2006. 47:563–566.
Article46. Ferroli P, Casazza M, Marras C, Mendola C, Franzini A, Broggi G. Cerebral cavernomas and seizures: A retrospective study on 163 patients who underwent pure lesionectomy. Neurol Sci. 2006. 26:390–394.
Article47. Cohen DS, Zubay GP, Goodman RR. Seizure outcome after lesionectomy for cavernous malformations. J Neurosurg. 1995. 83:237–242.
Article48. Cappabianca P, Alfieri A, Maiuri F, Mariniello G, Cirillo S, de Divitiis E. Supratentorial cavernous malformations and epilepsy: Seizure outcome after lesionectomy on a series of 35 patients. Clin Neurol Neurosurg. 1997. 99:179–183.
Article49. Hammen T, Romstock J, Dorfler A, Kerling F, Buchfelder M, Stefan H. Prediction of postoperative outcome with special respect to removal of hemosiderin fringe: A study in patients with cavernous haemangiomas associated with symptomatic epilepsy. Seizure. 2007. 16:248–253.
Article50. Maehara T, Nariai T, Arai N, Kawai K, Shimizu H, Ishii K, et al. Usefulness of [11C]methionine PET in the diagnosis of dysembryoplastic neuroepithelial tumor with temporal lobe epilepsy. Epilepsia. 2004. 45:41–45.
Article51. Rosenberg DS, Demarquay G, Jouvet A, Le Bars D, Streichenberger N, Sindou M, et al. [11C]-methionine PET: Dysembryoplastic neuroepithelial tumours compared with other epileptogenic brain neoplasms. J Neurol Neurosurg Psychiatry. 2005. 76:1686–1692.
Article52. Richardson MP, Hammers A, Brooks DJ, Duncan JS. Benzodiazepine-GABA(A) receptor binding is very low in dysembryoplastic neuroepithelial tumor: A PET study. Epilepsia. 2001. 42:1327–1334.
Article53. Prayson RA, Estes ML, Morris HH. Coexistence of neoplasia and cortical dysplasia in patients presenting with seizures. Epilepsia. 1993. 34:609–615.
Article54. Ferreira FT, Kobayashi E, Lopes-Cendes I, Cendes F. Structural abnormalities are similar in familial and nonfamilial mesial temporal lobe epilepsy. Can J Neurol Sci. 2004. 31:368–372.
Article55. Valenti MP, Froelich S, Armspach JP, Chenard MP, Dietemann JL, Kerhli P, et al. Contribution of SISCOM imaging in the presurgical evaluation of temporal lobe epilepsy related to dysembryoplastic neuroepithelial tumors. Epilepsia. 2002. 43:270–276.
Article56. Bautista JF, Foldvary-Schaefer N, Bingaman WE, Luders HO. Focal cortical dysplasia and intractable epilepsy in adults: Clinical, EEG, imaging, and surgical features. Epilepsy Res. 2003. 55:131–136.
Article57. Fauser S, Schulze-Bonhage A, Honegger J, Carmona H, Huppertz HJ, Pantazis G, et al. Focal cortical dysplasias: Surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain. 2004. 127:2406–2418.
Article58. Bernasconi A. Quantitative MR imaging of the neocortex. Neuroimaging Clin N Am. 2004. 14:425–436.
Article59. Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000. 11:805–821.
Article60. Keller SS, Wilke M, Wieshmann UC, Sluming VA, Roberts N. Comparison of standard and optimized voxelbased morphometry for analysis of brain changes associated with temporal lobe epilepsy. Neuroimage. 2004. 23:860–868.
Article61. Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004. 23:717–723.
Article62. Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: A volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003. 126:462–469.
Article63. Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW. Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2006. 47:900–907.
Article64. Colliot O, Bernasconi N, Khalili N, Antel SB, Naessens V, Bernasconi A. Individual voxel-based analysis of gray matter in focal cortical dysplasia. Neuroimage. 2006. 29:162–171.
Article65. Bonilha L, Montenegro MA, Rorden C, Castellano G, Guerreiro MM, Cendes F, et al. Voxel-based morphometry reveals excess gray matter concentration in patients with focal cortical dysplasia. Epilepsia. 2006. 47:908–915.
Article66. Rugg-Gunn FJ, Boulby PA, Symms MR, Barker GJ, Duncan JS. Whole-brain T2 mapping demonstrates occult abnormalities in focal epilepsy. Neurology. 2005. 64:318–325.
Article67. Salmenpera TM, Symms MR, Rugg-Gunn FJ, Boulby PA, Free SL, Barker GJ, et al. Evaluation of quantitative magnetic resonance imaging contrasts in MRInegative refractory focal epilepsy. Epilepsia. 2007. 48:229–237.
Article68. Won HJ, Chang KH, Cheon JE, Kim HD, Lee DS, Han MH, et al. Comparison of MR imaging with PET and ictal SPECT in 118 patients with intractable epilepsy. AJNR Am J Neuroradiol. 1999. 20:593–599.69. Gaillard WD. Wyllie E, Gupta A, Lachhwani D, editors. Metabolic and functional imaging. The treatment of epilepsy. 2006. 4th ed. Philadelphia: Lippincott Williams & Wilkins;1041–1058.70. Choi JY, Kim SJ, Hong SB, Seo DW, Hong SC, Kim BT, et al. Extratemporal hypometabolism on FDG PET intemporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging. 2003. 30:581–587.
Article71. Vinton AB, Carne R, Hicks RJ, Desmond PM, Kilpatrick C, Kaye AH, et al. The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain. 2007. 130:548–560.
Article72. Carne RP, O'Brien TJ, Kilpatrick CJ, MacGregor LR, Hicks RJ, Murphy MA, et al. MRI-negative PET-positive temporal lobe epilepsy: A distinct surgically remediable syndrome. Brain. 2004. 127:2276–2285.
Article73. Koepp MJ, Hammers A, Labbe C, Woermann FG, Brooks DJ, Duncan JS. 11C-flumazenil PET in patients with refractory temporal lobe epilepsy and normal MRI. Neurology. 2000. 54:332–339.
Article74. Hammers A, Koepp MJ, Richardson MP, Hurlemann R, Brooks DJ, Duncan JS. Grey and white matter flumazenil binding in neocortical epilepsy with normal MRI. A PET study of 44 patients. Brain. 2003. 126:1300–1318.
Article75. Hammers A, Koepp MJ, Brooks DJ, Duncan JS. Periventricular white matter flumazenil binding and postoperative outcome in hippocampal sclerosis. Epilepsia. 2005. 46:944–948.
Article76. Yun CH, Lee SK, Lee SY, Kim KK, Jeong SW, Chung CK. Prognostic factors in neocortical epilepsy surgery: Multivariate analysis. Epilepsia. 2006. 47:574–579.
Article77. Lee SK, Lee SY, Kim KK, Hong KS, Lee DS, Chung CK. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005. 58:525–532.
Article78. Van Paesschen W. Ictal SPECT. Epilepsia. 2004. 45:Suppl 4. S35–S40.
Article79. O'Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT coregistered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998. 50:445–454.80. Lee SK, Lee SY, Yun CH, Lee HY, Lee JS, Lee DS. Ictal SPECT in neocortical epilepsies: Clinical usefulness and factors affecting the pattern of hyperperfusion. Neuroradiology. 2006. 48:678–684.
Article81. O'Brien TJ, So EL, Mullan BP, Cascino GD, Hauser MF, Brinkmann BH, et al. Subtraction peri-ictal SPECT is predictive of extratemporal epilepsy surgery outcome. Neurology. 2000. 55:1668–1677.82. O'Brien TJ, So EL, Cascino GD, Hauser MF, Marsh WR, Meyer FB, et al. Subtraction SPECT coregistered to MRI in focal malformations of cortical development: Localization of the epileptogenic zone in epilepsy surgery candidates. Epilepsia. 2004. 45:367–376.83. Dupont P, Van Paesschen W, Palmini A, Ambayi R, Van Loon J, Goffin J, et al. Ictal perfusion patterns associated with single MRI-visible focal dysplastic lesions: Implications for the noninvasive delineation of the epileptogenic zone. Epilepsia. 2006. 47:1550–1557.
Article84. Medina LS, Bernal B, Dunoyer C, Cervantes L, Rodriguez M, Pacheco E, et al. Seizure disorders: Functional MR imaging for diagnostic evaluation and surgical treatment--prospective study. Radiology. 2005. 236:247–253.
Article85. Jack CR Jr, Thompson RM, Butts RK, Sharbrough FW, Kelly PJ, Hanson DP, et al. Sensory motor cortex: Correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology. 1994. 190:85–92.
Article86. Puce A, Constable RT, Luby ML, McCarthy G, Nobre AC, Spencer DD, et al. Functional magnetic resonance imaging of sensory and motor cortex: Comparison with electrophysiological localization. J Neurosurg. 1995. 83:262–270.
Article87. Lehericy S, Duffau H, Cornu P, Capelle L, Pidoux B, Carpentier A, et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: Comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg. 2000. 92:589–598.
Article88. Yetkin FZ, Mueller WM, Morris GL, McAuliffe TL, Ulmer JL, Cox RW, et al. Functional MR activation correlated with intraoperative cortical mapping. AJNR Am J Neuroradiol. 1997. 18:1311–1315.89. Rabin ML, Narayan VM, Kimberg DY, Casasanto DJ, Glosser G, Tracy JI, et al. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain. 2004. 127:2286–2298.
Article90. Richardson MP, Strange BA, Thompson PJ, Baxendale SA, Duncan JS, Dolan RJ. Pre-operative verbal memory fMRI predicts post-operative memory decline after left temporal lobe resection. Brain. 2004. 127:2419–2426.
Article91. Richardson MP, Strange BA, Duncan JS, Dolan RJ. Memory fMRI in left hippocampal sclerosis: Optimizing the approach to predicting postsurgical memory. Neurology. 2006. 66:699–705.
Article92. Janszky J, Jokeit H, Kontopoulou K, Mertens M, Ebner A, Pohlmann-Eden B, et al. Functional MRI predicts memory performance after right mesiotemporal epilepsy surgery. Epilepsia. 2005. 46:244–250.
Article93. Lehericy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, et al. Functional MR evaluation of temporal and frontal language dominance compared with the wada test. Neurology. 2000. 54:1625–1633.
Article94. FitzGerald DB, Cosgrove GR, Ronner S, Jiang H, Buchbinder BR, Belliveau JW, et al. Location of language in the cortex: A comparison between functional MR imaging and electrocortical stimulation. AJNR Am J Neuroradiol. 1997. 18:1529–1539.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Presurgical Invasive Study in Intractable Epilepsy: Intracranial Electrode and Brain Mapping
- Clinical and neuroimaging characteristics in patients with secondary bilateral synchrony (SBS) on EEG
- Epilepsy Surgery I
- Surgical Management of Medically Intractable Epilepsy
- Multimodal neuroimaging in presurgical evaluation of childhood epilepsy