J Breast Cancer.
2010 Jun;13(2):231-235. 10.4048/jbc.2010.13.2.231.
Docetaxel-induced Severe Fluid Retention in a Breast Cancer Patient: A Case Report
- Affiliations
-
- 1Department of Surgery, Yeungnam University College of Medicine, Daegu, Korea. crystallee@medical.yu.ac.kr
- KMID: 2286551
- DOI: http://doi.org/10.4048/jbc.2010.13.2.231
Abstract
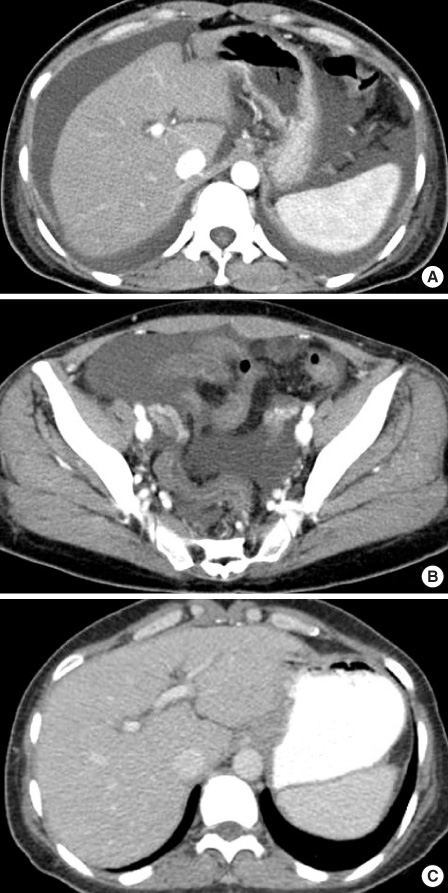
- Among many adverse effects of docetaxel, fluid retention is a well recognized, cumulative side effect, but severe fluid retention is rare. We report here on a case of docetaxel-induced severe fluid retention with peripheral edema, pleural effusion, severe ascites and pericardial effusion in a 41-year-old woman. She had been treated with 3 cycles of docetaxel 9 days previously and she was admitted to our hospital due to abdominal distention and mild dyspnea. Radiologic studies revealed pleural effusion, severe ascites and a small pericardial effusion. Diuretics were given for 21 days. The pleural effusion was resolved after treatment with diuretics for 2 days, but the ascites wasn't resolved until 14 days of diuretics. After treatment with diuretics for 21 days, all the symptoms of the patient were completely resolved. Early detection is mandatory and diuretics are very effective for patient suffering with docetaxel-induced severe fluid retention.
Keyword
MeSH Terms
Figure
Reference
-
1. Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999. 10:947–959.
Article2. Valero V, Holmes FA, Walters RS, Theriault RL, Esparza L, Fraschini G, et al. Phase II trial of docetaxel: a new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol. 1995. 13:2886–2894.
Article3. Piccart MJ, Klijn J, Paridaens R, Nooij M, Mauriac L, Coleman R, et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J Clin Oncol. 1997. 15:3149–3155.
Article4. Baker J, Ajani J, Scotte F, Winther D, Martin M, Aapro MS, et al. Docetaxel-related side effects and their management. Eur J Oncol Nurs. 2009. 13:49–59.
Article5. Ferraresi V, Milella M, Vaccaro A, D'Ottavio AM, Papaldo P, Nistico C, et al. Toxicity and activity of docetaxel in anthracycline-pretreated breast cancer patients: a phase II study. Am J Clin Oncol. 2000. 23:132–139.
Article6. Ryu DW, Jun CW, Lee CH. Neoadjuvant chemotherapy with docetaxel and adriamycin in breast cancer: clincopathologic factors influencing to response rate. J Breast Cancer. 2008. 11:89–94.
Article7. Tomiak E, Piccart MJ, Kerger J, Lips S, Awada A, de Valeriola D, et al. Phase I study of docetaxel administered as a 1-hour intravenous infusion on a weekly basis. J Clin Oncol. 1994. 12:1458–1467.
Article8. Semb KA, Aamdal S, Oian P. Capillary protein leak syndrome appears to explain fluid retention in cancer patients who receive docetaxel treatment. J Clin Oncol. 1998. 16:3426–3432.
Article9. Fossella FV, Lee JS, Murphy WK, Lippman SM, Calayag M, Pang A, et al. Phase II study of docetaxel for recurrent or metastatic non-small-cell lung cancer. J Clin Oncol. 1994. 12:1238–1244.
Article10. McKeage K, Keam SJ. Docetaxel in hormone-refractory metastatic prostate cancer. Drugs. 2005. 65:2287–2294.
Article11. Massacesi C, Marcucci F, Rocchi MB, Mazzanti P, Pilone A, Bonsignori M. Factors predicting docetaxel-related toxicity: experience at a single institution. J Chemother. 2004. 16:86–93.
Article12. Pronk LC, van Putten WL, van Beurden V, de Boer-Dennert M, Stoter G, Verweij J. The venotonic drug hydroxyethylrutosiden does not prevent or reduce docetaxel-induced fluid retention: results of a comparative study. Cancer Chemother Pharmacol. 1999. 43:173–177.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Scleroderma-like Cutaneous Lesions Induced by Docetaxel in a Patient with Breast Cancer
- The Efficacy and Safety of Docetaxel in Patients with Anthracychne pretreated Metastatic Breast Cancer: A Multicenter Phase II Study
- A Case of Subungal Abscess And Onycholysis Related to Docetaxel
- A Case of Localized Erythematous Dermatitis on the Site of a Docetaxel Injection
- Docetaxel-Induced Onycholysis: The Role of Subungual Hemorrhage and Suppuration