J Breast Cancer.
2012 Dec;15(4):474-477. 10.4048/jbc.2012.15.4.474.
A Case of Pseudo-Meigs' Syndrome Associated with Ovarian Metastases from Breast Cancer
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Wakayama Medical University School of Medicine, Wakayama, Japan. soura@wakayama-med.ac.jp
- 2Department of Clinical and Surgical Pathology, Wakayama Medical University School of Medicine, Wakayama, Japan.
- KMID: 2286447
- DOI: http://doi.org/10.4048/jbc.2012.15.4.474
Abstract
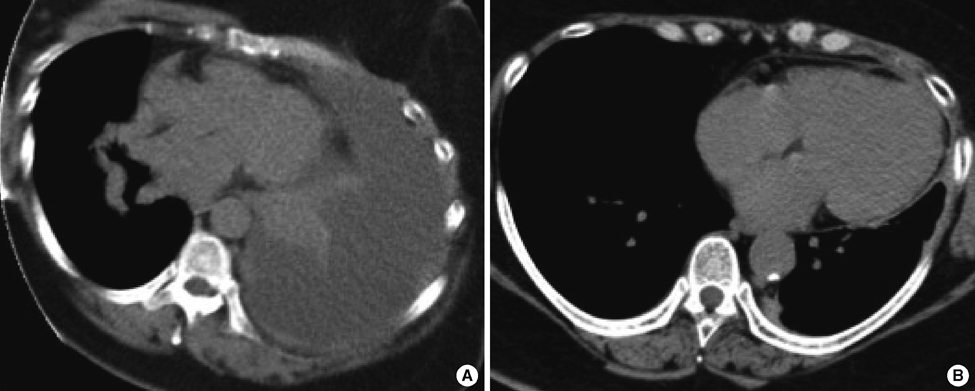
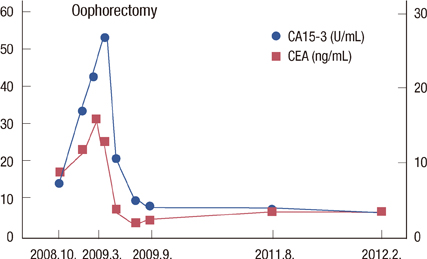
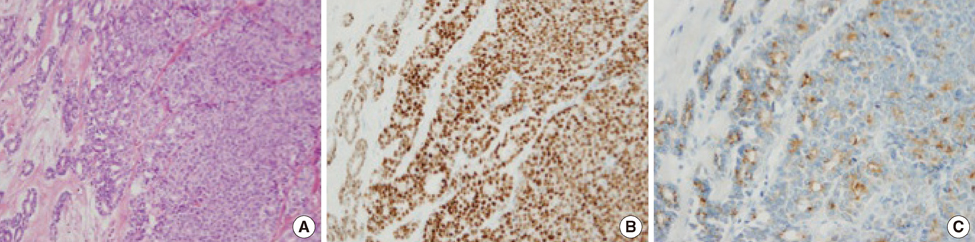
- A 54-year-old woman with long-lasting pleural effusion developed abdominal distention due to ascites from bilateral ovarian tumors. The patient had undergone breast-conserving surgery and axillary lymph node dissection for left breast cancer in October 2000, and had developed left pleural effusion in July 2006. Cytological examination of the pleural effusion found no malignant cells. Thoracic drainage with intrathoracic administration of OK-432 (Picibanil) had failed to control the pleural effusion. Positron emission tomography taken at the abdominal distention showed bilateral ovarian tumors. After failure to control the ascites with systemic and intra-abdominal chemotherapy, bilateral oophorectomy resulted in normalization of elevated serum tumor-marker levels and the disappearance of both the ascites and pleural effusions (i.e., pseudo-Meigs' syndrome). Pathological examination showed the tumors to be estrogen receptor-positive metastatic ovarian tumors from her breast cancer. The patient remained well with no further recurrence for 40 months under aromatase inhibitor therapy.
MeSH Terms
Figure
Reference
-
1. Meigs JV, Cass JW. Fibroma of the ovary with ascites and hydrothorax. Am J Obstet Gynecol. 1937. 33:249–267.
Article2. Rhoads JE, Terrell AW. Ovarian fibroma with ascites and hydrothorax (Meigs's syndrome): report of a case. JAMA. 1937. 109:1684–1687.
Article3. Meigs JV, Armstrong SH, Hamilton HH. A further contribution to the syndrome of fibroma of the ovary with fluid in the abdomen and chest, Meigs' syndrome. Am J Obstet Gynecol. 1943. 46:19–37.
Article4. Meigs JV. Pelvic tumors other than fibromas of the ovary with ascites and hydrothorax. Obstet Gynecol. 1954. 3:471–486.
Article5. Fujii M, Okino M, Fujioka K, Yamashita K, Hamano K. Pseudo-Meigs' syndrome caused by breast cancer metastasis to both ovaries. Breast Cancer. 2006. 13:344–348.
Article6. Kawakubo N, Okido M, Tanaka R, Mitsugi K, Fukuhara M, Aishima S, et al. Pseudo-Meigs' syndrome associated with breast cancer metastasis to both ovaries: report of a case. Surg Today. 2010. 40:1148–1151.
Article7. O'Flanagan SJ, Tighe BF, Egan TJ, Delaney PV. Meigs' syndrome and pseudo-Meigs' syndrome. J R Soc Med. 1987. 80:252–253.8. Abramov Y, Anteby SO, Fasouliotis SJ, Barak V. The role of inflammatory cytokines in Meigs' syndrome. Obstet Gynecol. 2002. 99:917–919.
Article9. Gagnon Y, Têtu B. Ovarian metastases of breast carcinoma. A clinicopathologic study of 59 cases. Cancer. 1989. 64:892–898.
Article10. Shiromizu K, Kawana T, Sugase M, Izumi R, Mizuno M. Experience with the treatment of metastatic ovarian carcinoma. Arch Gynecol Obstet. 1988. 243:111–114.
Article11. Tsubono M, Kaneko I, Kii E, Tanaka T, Murata T, Kamimura K, et al. Weekly paclitaxel administration and intraabdominal CBDCA injection possibly beneficial treatment for recurrent breast cancer associated with metastatic ovarian cancer and peritoneal dissemination after operation: a case report. Gan To Kagaku Ryoho. 2005. 32:365–369.12. Ishibashi M, Nakayama K, Oride A, Yeasmin S, Katagiri A, Iida K, et al. A case of PEP(BEP)-resistant ovarian dysgerminoma successfully treated by VeIP therapy. Gan To Kagaku Ryoho. 2009. 36:513–517.13. Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998. 339:974–984.
Article14. Hortobagyi GN. Can we cure limited metastatic breast cancer? J Clin Oncol. 2002. 20:620–623.
Article15. Singletary SE, Walsh G, Vauthey JN, Curley S, Sawaya R, Weber KL, et al. A role for curative surgery in the treatment of selected patients with metastatic breast cancer. Oncologist. 2003. 8:241–251.
Article