Korean J Physiol Pharmacol.
2013 Dec;17(6):553-558. 10.4196/kjpp.2013.17.6.553.
Long-Term Potentiation of Excitatory Synaptic Strength in Spinothalamic Tract Neurons of the Rat Spinal Cord
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 110-799, Korea.
- 2Department of Physiology, Jeju National University College of Medicine, Jeju 690-756, Korea. joominp@jejunu.ac.kr
- KMID: 2285484
- DOI: http://doi.org/10.4196/kjpp.2013.17.6.553
Abstract
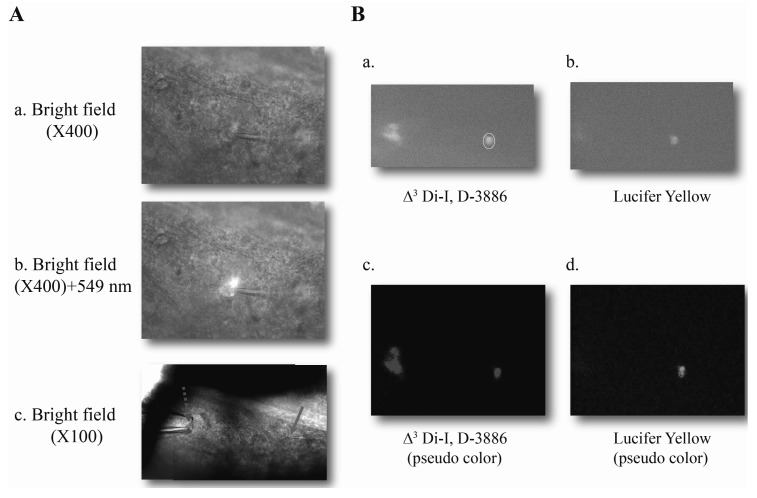
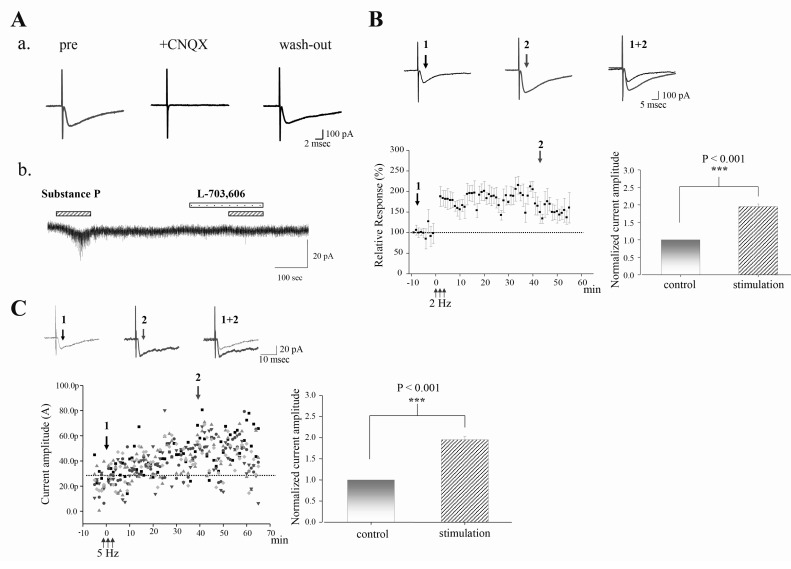
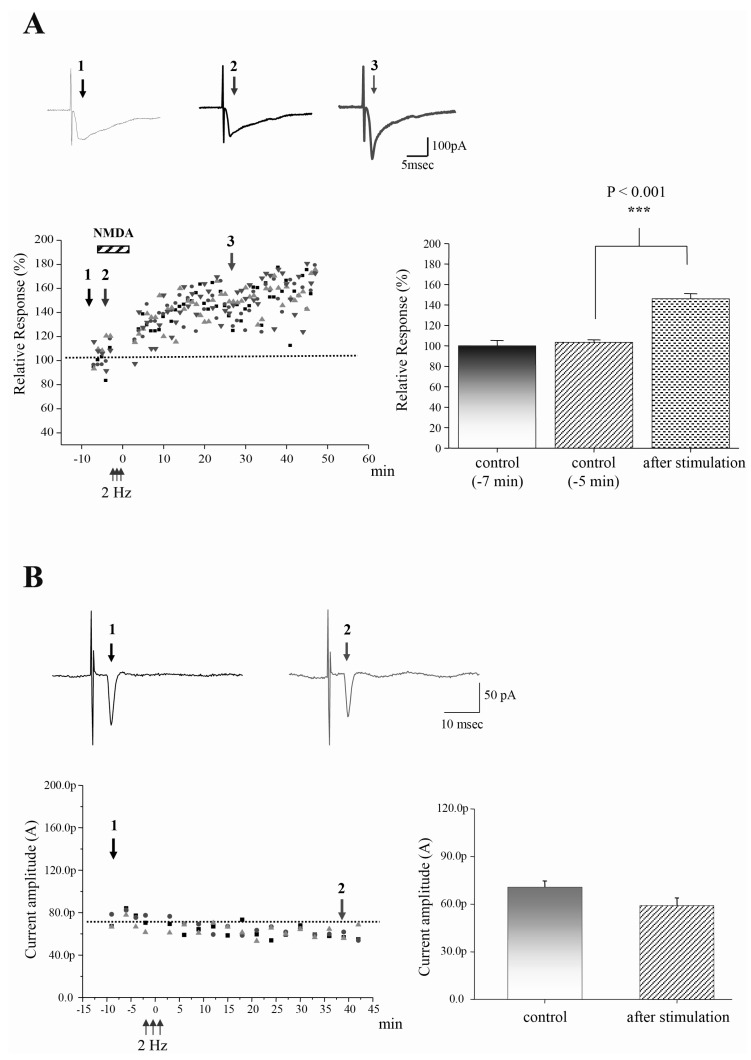
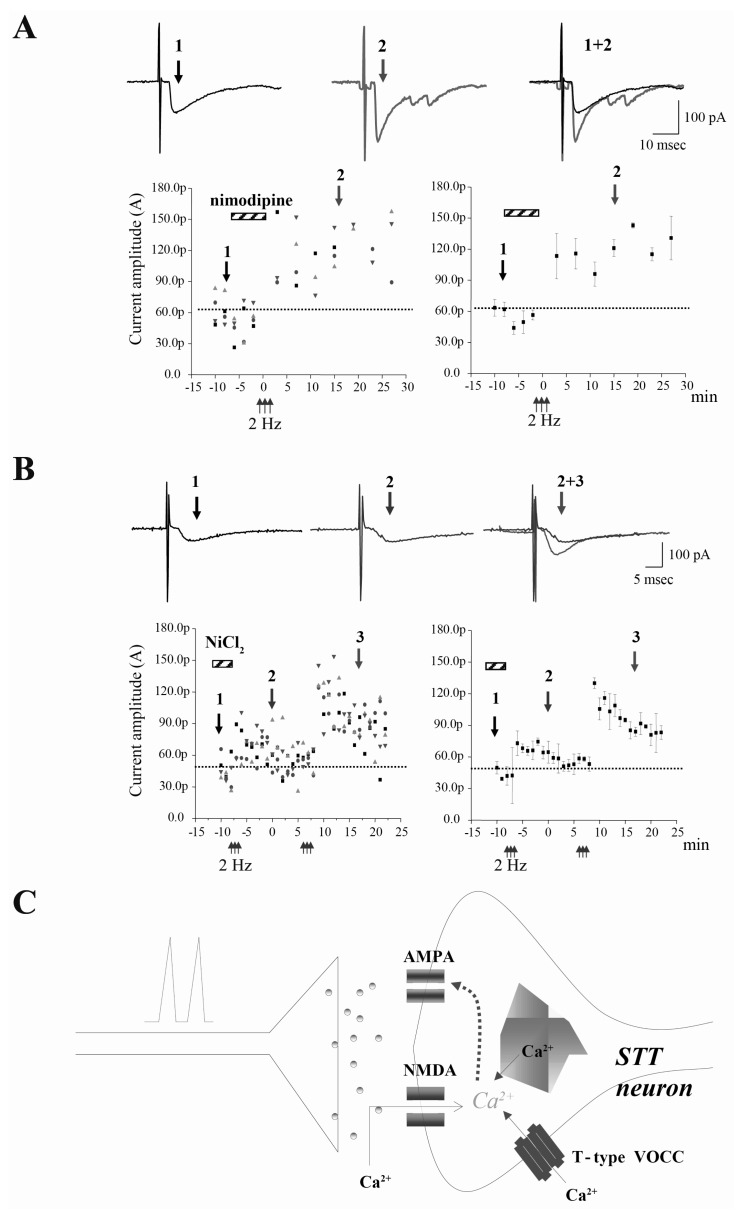
- Spinal dorsal horn nociceptive neurons have been shown to undergo long-term synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD). Here, we focused on the spinothalamic tract (STT) neurons that are the main nociceptive neurons projecting from the spinal cord to the thalamus. Optical technique using fluorescent dye has made it possible to identify the STT neurons in the spinal cord. Evoked fast mono-synaptic, excitatory postsynaptic currents (eEPSCs) were measured in the STT neurons. Time-based tetanic stimulation (TBS) was employed to induce long-term potentiation (LTP) in the STT neurons. Coincident stimulation of both pre- and postsynaptic neurons using TBS showed immediate and persistent increase in AMPA receptor-mediated EPSCs. LTP can also be induced by postsynaptic spiking together with pharmacological stimulation using chemical NMDA. TBS-induced LTP observed in STT neurons was blocked by internal BAPTA, or Ni2+, a T-type VOCC blocker. However, LTP was intact in the presence of L-type VOCC blocker. These results suggest that long-term plastic change of STT neurons requires NMDA receptor activation and postsynaptic calcium but is differentially sensitive to T-type VOCCs.
MeSH Terms
-
alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
Animals
Calcium
Depression
Egtazic Acid
Excitatory Postsynaptic Potentials
Horns
Long-Term Potentiation*
N-Methylaspartate
Neurons*
Nociceptors
Plastics
Rats*
Spinal Cord*
Spinothalamic Tracts*
Thalamus
Calcium
Egtazic Acid
N-Methylaspartate
Plastics
alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
Figure
Cited by 2 articles
-
Long-term Synaptic Plasticity: Circuit Perturbation and Stabilization
Joo Min Park, Sung-Cherl Jung, Su-Yong Eun
Korean J Physiol Pharmacol. 2014;18(6):457-460. doi: 10.4196/kjpp.2014.18.6.457.NgR1 Expressed in P19 Embryonal Carcinoma Cells Differentiated by Retinoic Acid Can Activate STAT3
Su In Lee, Jieun Yun, Ji-Young Baek, Yun-Ji Jeong, Jin-Ah Kim, Jong Soon Kang, Sun Hong Park, Sang Kyum Kim, Song-Kyu Park
Korean J Physiol Pharmacol. 2015;19(2):105-109. doi: 10.4196/kjpp.2015.19.2.105.
Reference
-
1. Rygh LJ, Tjølsen A, Hole K, Svendsen F. Cellular memory in spinal nociceptive circuitry. Scand J Psychol. 2002; 43:153–159. PMID: 12004953.
Article2. Chen SR, Hu YM, Chen H, Pan HL. Calcineurin inhibitor induces pain hypersensitivity by potentiating pre- and postsynaptic NMDA receptor activity in spinal cords. J Physiol. 2013; [Epub ahead of print].
Article3. Liu X, Sandkühler J. Characterization of long-term potentiation of C-fiber-evoked potentials in spinal dorsal horn of adult rat: essential role of NK1 and NK2 receptors. J Neurophysiol. 1997; 78:1973–1982. PMID: 9325365.
Article4. Liu XG, Sandkühler J. Long-term potentiation of C-fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-D-aspartic acid receptor blockage. Neurosci Lett. 1995; 191:43–46. PMID: 7659287.
Article5. Randić M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993; 13:5228–5241. PMID: 8254370.6. Rygh LJ, Svendsen F, Hole K, Tjølsen A. Natural noxious stimulation can induce long-term increase of spinal nociceptive responses. Pain. 1999; 82:305–310. PMID: 10488682.
Article7. Li JL, Ding YQ, Shigemoto R, Mizuno N. Distribution of trigeminothalamic and spinothalamic-tract neurons showing substance P receptor-like immunoreactivity in the rat. Brain Res. 1996; 719:207–212. PMID: 8782883.
Article8. Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998; 18:10464–10472. PMID: 9852584.
Article9. Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. TINS. 1992; 15:96–103. PMID: 1373925.
Article10. Pagadala P, Park CK, Bang S, Xu ZZ, Xie RG, Liu T, Han BX, Tracey WD Jr, Wang F, Ji RR. Loss of NR1 subunit of NMDARs in primary sensory neurons leads to hyperexcitability and pain hypersensitivity: involvement of Ca2+-activated small conductance potassium channels. J Neurosci. 2013; 33:13425–13430. PMID: 23946399.11. Traub RJ. Spinal modulation of the induction of central sensitization. Brain Res. 1997; 778:34–42. PMID: 9462875.
Article12. Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann N Y Acad Sci. 2001; 933:142–156. PMID: 12000017.
Article14. Dougherty PM, Willis WD. Enhancement of spinothalamic neuron responses to chemical and mechanicalstimuli following combined micro-iontophoretic application of N-methyl-D-aspartic acid and substance P. Pain. 1991; 47:85–93. PMID: 1722895.15. Polgár E, Al-Khater KM, Shehab S, Watanabe M, Todd AJ. Large projection neurons in lamina I of the rat spinal cord that lack the neurokinin 1 receptor are densely innervated by VGLUT2-containing axons and possess GluR4-containing AMPA receptors. J Neurosci. 2008; 28:13150–13160. PMID: 19052206.16. Willis WD. Mechanisms of central sensitization of nociceptive dorsal horn neurons. In : Patterson MM, Grau JW, editors. Spinal Cord Plasticity: alterations in Reflex Function. Boston: Kluwer Academic Publishers;2001. p. 127–161.17. Gerber G, Youn DH, Hsu CH, Isaev D, Randić M. Spinal dorsal horn synaptic plasticity: involvement of group I metabotropic glutamate receptors. Prog Brain Res. 2000; 129:115–134. PMID: 11098685.
Article18. Sandkühler J, Chen JG, Cheng G, Randić M. Low-frequency stimulation of afferent Adelta-fibers induces long-term depression at primary afferent synapses with substantia gelatinosa neurons in the rat. J Neurosci. 1997; 17:6483–6491. PMID: 9236256.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peripheral Nerve Injury Alters Excitatory and Inhibitory Synaptic Transmission in Rat Spinal Cord Substantia Gelatinosa
- Molecular Mechanisms Involved in Depotentiation and Their Relevance to Schizophrenia
- Changes in Synaptic Transmission and Long-term Potentiation Induction as a Possible Mechanism for Learning Disability in an Animal Model of Multiple Sclerosis
- Long-term Synaptic Plasticity: Circuit Perturbation and Stabilization
- The Role of NMDA Receptor in Learning and Memory