Korean J Physiol Pharmacol.
2013 Dec;17(6):537-546. 10.4196/kjpp.2013.17.6.537.
Potassium Currents in Isolated Deiters' Cells of Guinea Pig
- Affiliations
-
- 1Department of Otolaryngology, Asan Medical Center and University of Ulsan College of Medicine, Seoul 138-736, Korea.
- 2Department of Otolaryngology, Kangwon National University College of Medicine, Chuncheon 200-701, Korea.
- 3Department of Physiology, Asan Medical Center and University of Ulsan College of Medicine, Seoul 138-736, Korea. leemch@amc.seoul.kr
- 4Department of Physiology, College of Medicine, Cardiovascular and Metabolic Disease Center, Inje University, Busan 614-735, Korea.
- KMID: 2285482
- DOI: http://doi.org/10.4196/kjpp.2013.17.6.537
Abstract
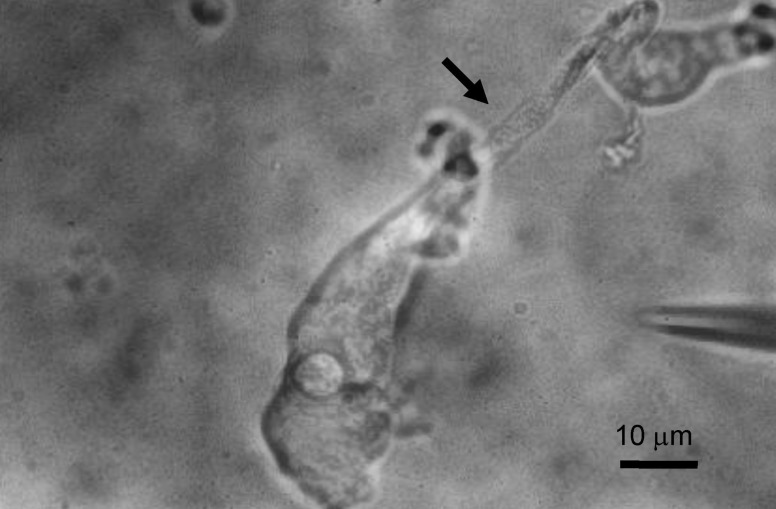
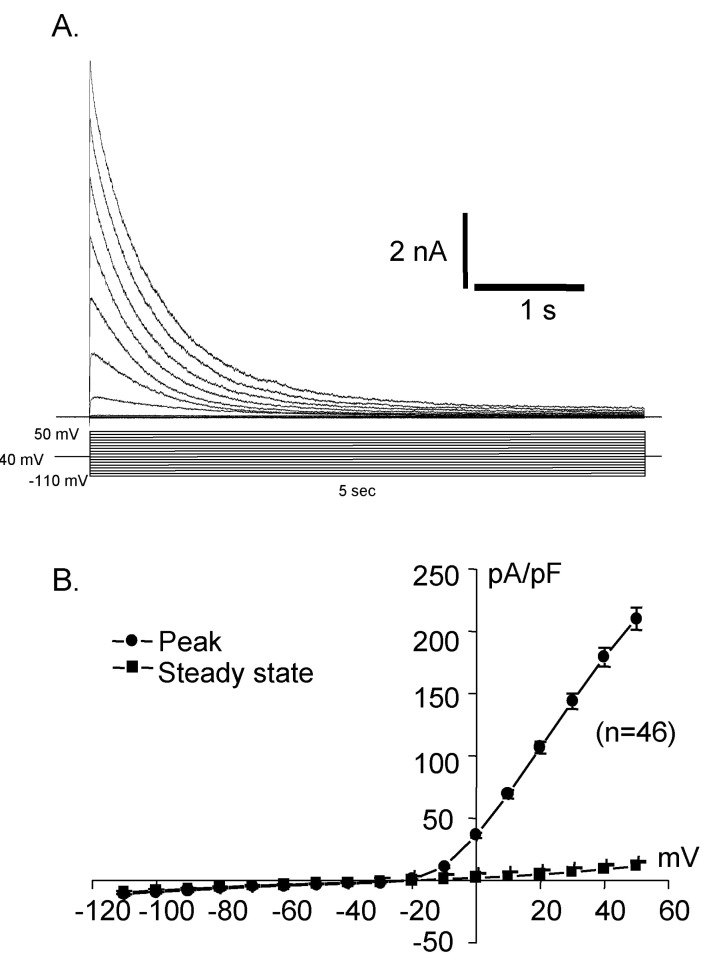
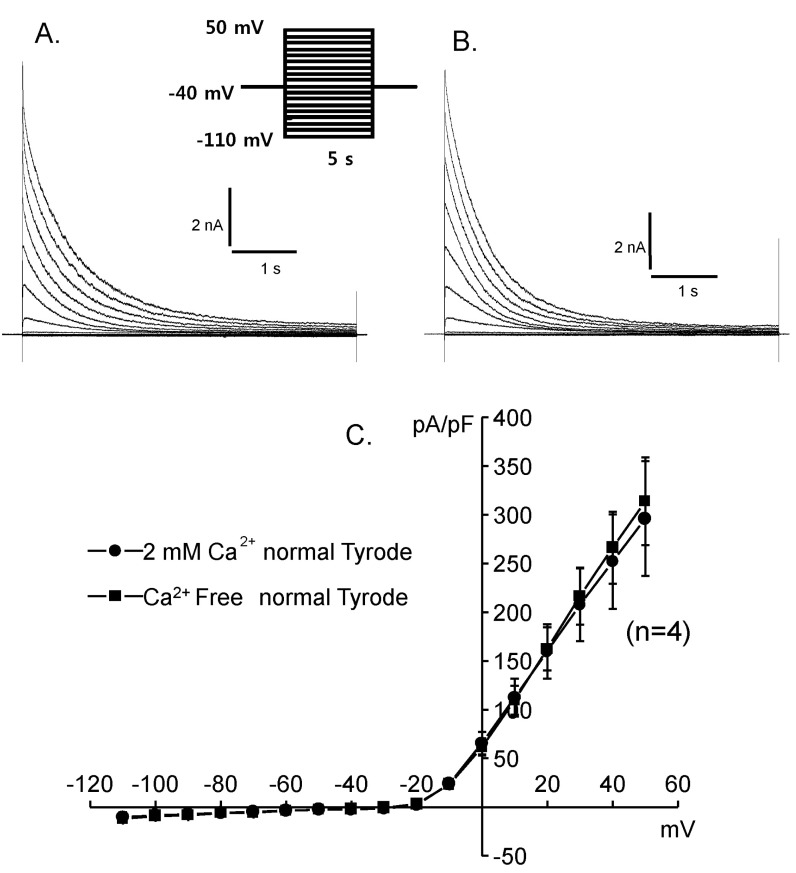
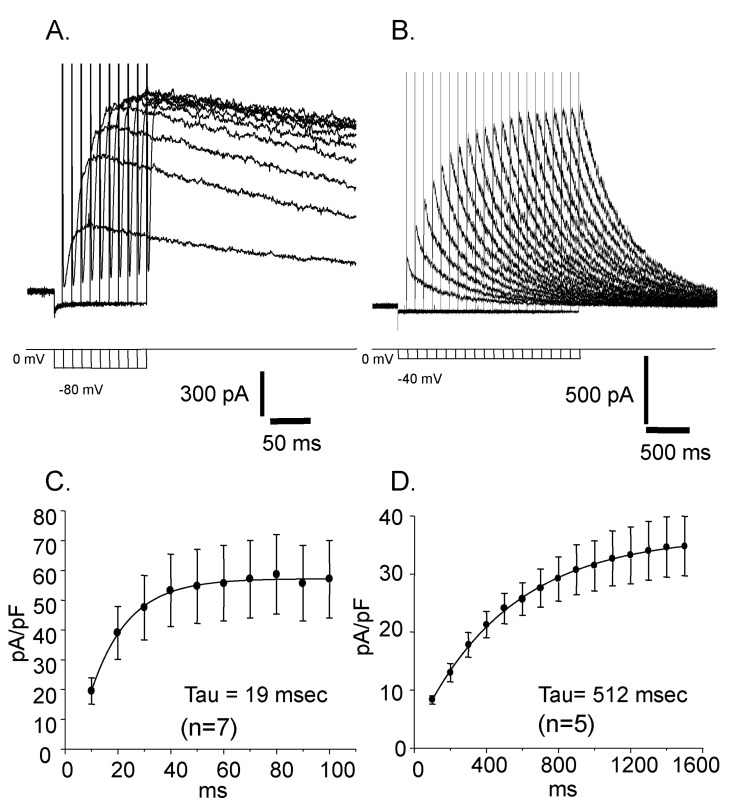
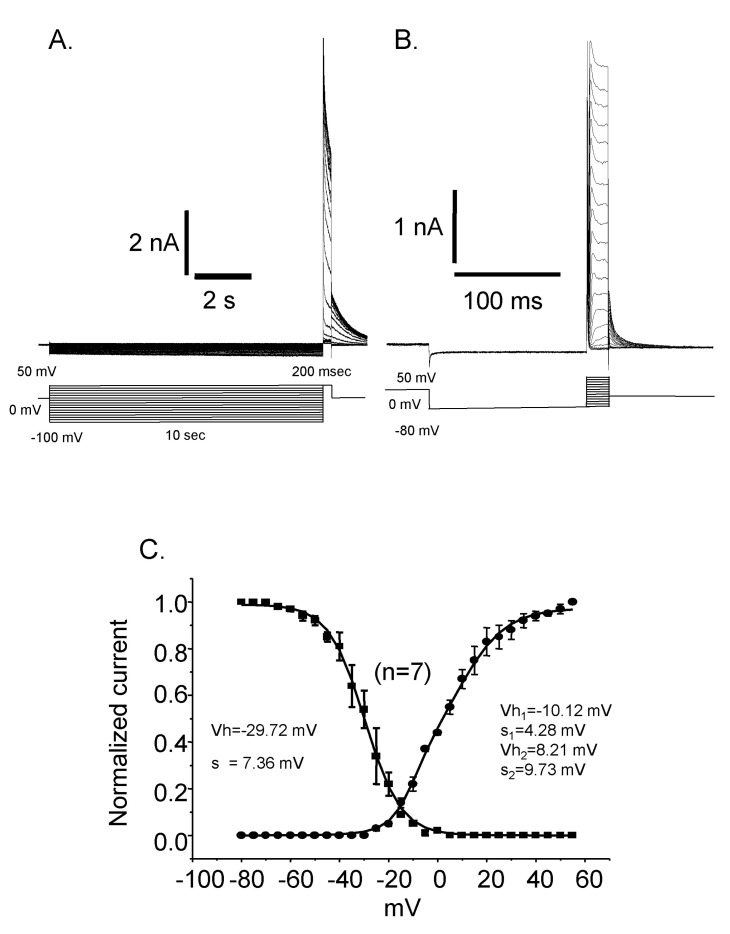
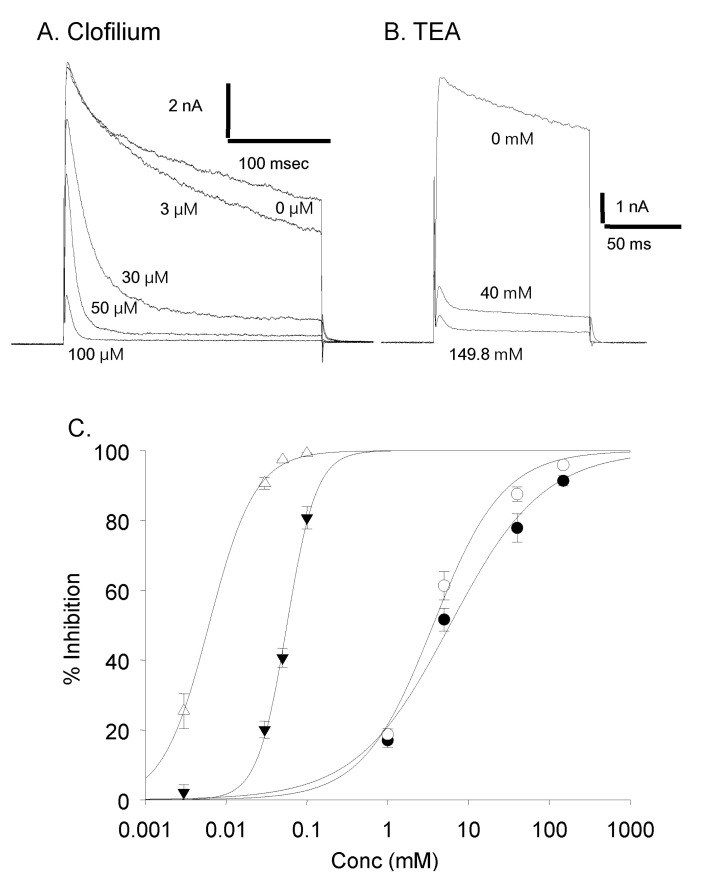
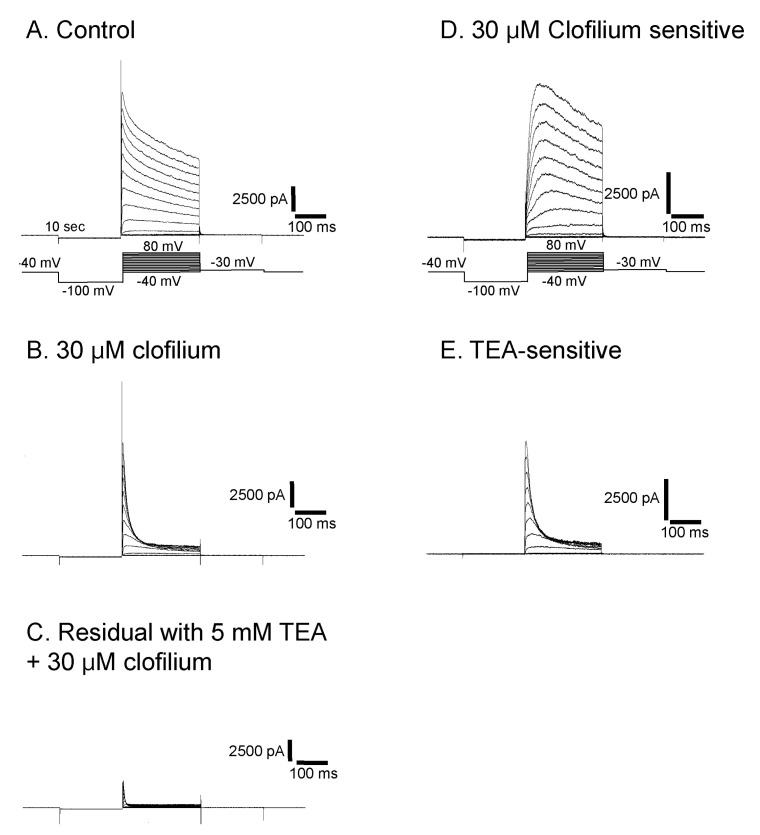
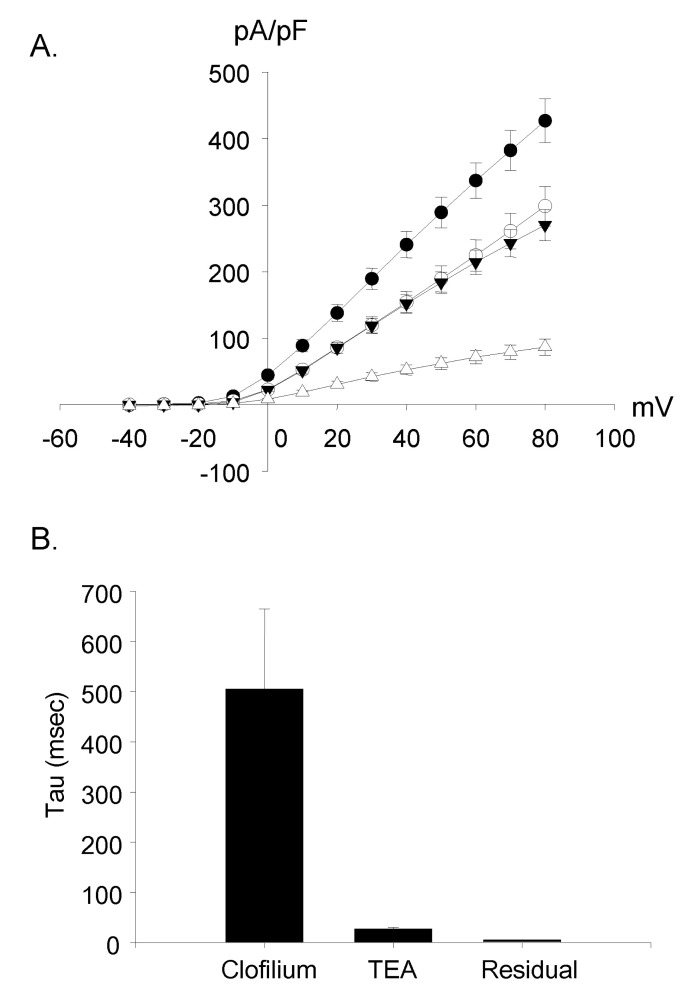
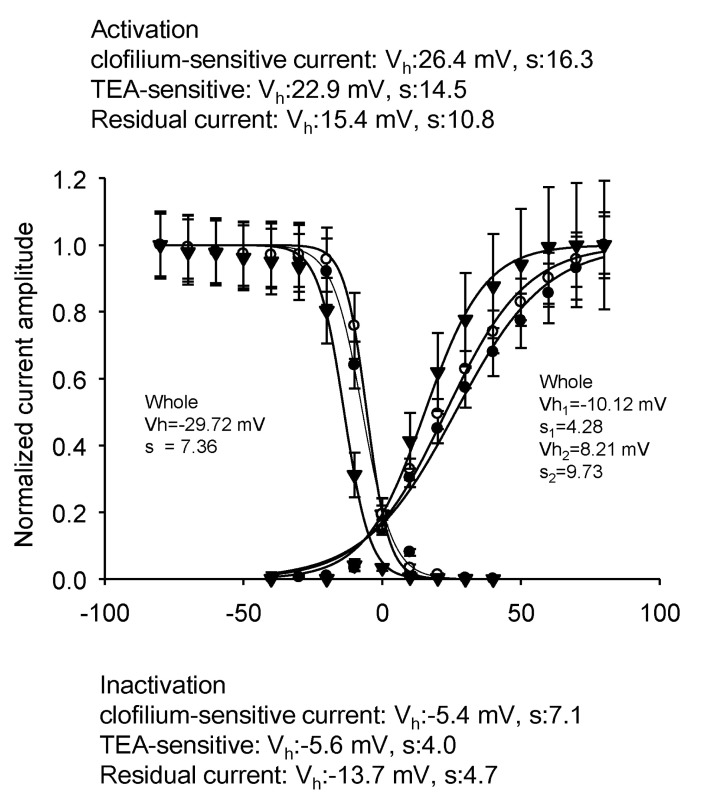
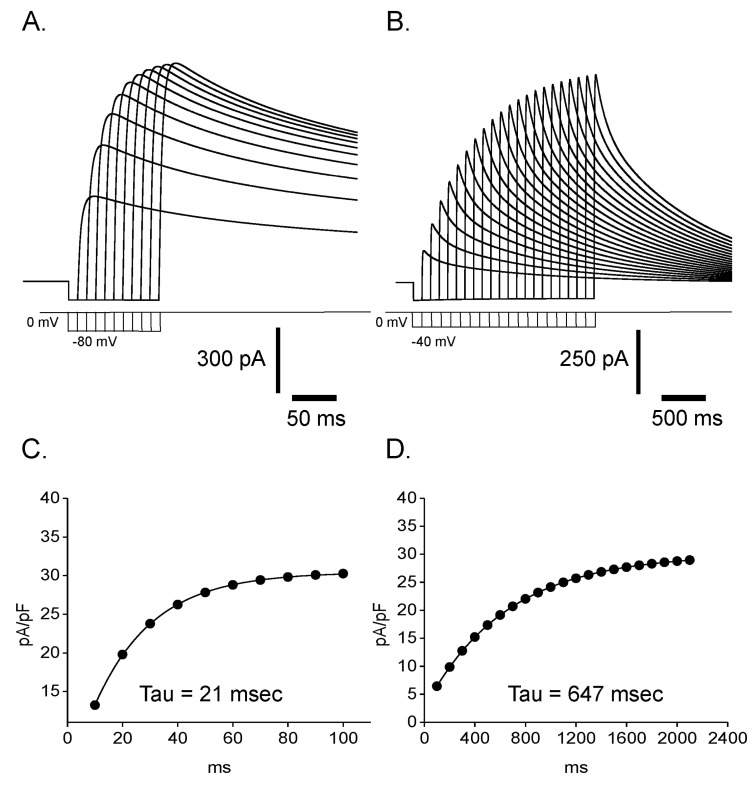
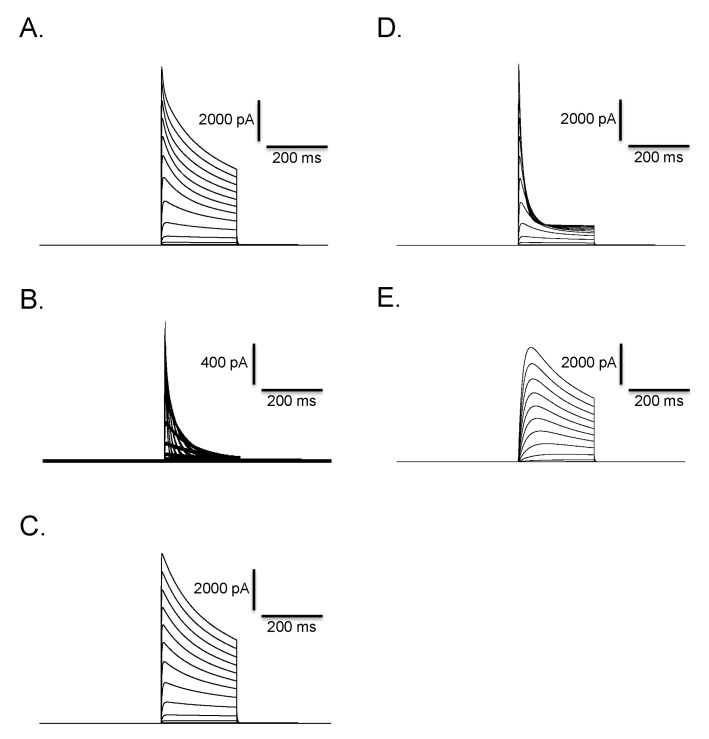
- Deiters' cells are the supporting cells in organ of Corti and are suggested to play an important role in biochemical and mechanical modulation of outer hair cells. We successfully isolated functionally different K+ currents from Deiters' cells of guinea pig using whole cell patch clamp technique. With high K+ pipette solution, depolarizing step pulses activated strongly outward rectifying currents which were dose-dependently blocked by clofilium, a class III anti-arrhythmic K+ channel blocker. The remaining outward current was transient in time course whereas the clofilium-sensitive outward current showed slow inactivation and delayed rectification. Addition of 5 mM tetraethylammonium (TEA) further blocked the remaining current leaving a very fast inactivating transient outward current. Therefore, at least three different types of K+ current were identified in Deiters' cells, such as fast activating and fast inactivating current, fast activating slow inactivating current, and very fast inactivating transient outward current. Physiological role of them needs to be established.
MeSH Terms
Figure
Reference
-
1. Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol. 1990; 301:443–460. PMID: 2262601.
Article2. Wright CG, Preston RE. Efferent nerve fibers associated with the outermost supporting cells of the organ of Corti in the guinea pig. Acta Otolaryngol. 1976; 82:41–47. PMID: 948984.
Article3. Burgess BJ, Adams JC, Nadol JB Jr. Morphologic evidence for innervation of Deiters' and Hensens cells in the guinea pig. Hear Res. 1997; 108:74–82. PMID: 9213124.
Article4. Matsunobu T, Chung JW, Schacht J. Acetylcholine-evoked calcium increases in Deiters' cells of the guinea pig cochlea suggest alpha9-like receptors. J Neurosci Res. 2001; 63:252–256. PMID: 11170174.5. Chung JW, Schacht J. ATP and nitric oxide modulate intracellular calcium in isolated pillar cells of the guinea pig cochlea. J Assoc Res Otolaryngol. 2001; 2:399–407. PMID: 11833612.
Article6. Matsunobu T, Schacht J. Nitric oxide/cyclic GMP pathway attenuates ATP-evoked intracellular calcium increase in supporting cells of the guinea pig cochlea. J Comp Neurol. 2000; 423:452–461. PMID: 10870085.
Article7. Bobbin RP. ATP-induced movement of the stalks of isolated cochlear Deiters' cells. Neuroreport. 2001; 12:2923–2926. PMID: 11588604.
Article8. Dulon D, Blanchet C, Laffon E. Photo-released intracellular Ca2+ evokes reversible mechanical responses in supporting cells of the guinea-pig organ of Corti. Biochem Biophys Res Commun. 1994; 201:1263–1269. PMID: 8024570.9. Fridberger A, Flock A, Ulfendahl M, Flock B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc Natl Acad Sci U S A. 1998; 95:7127–7132. PMID: 9618550.10. Nenov AP, Chen C, Bobbin RP. Outward rectifying potassium currents are the dominant voltage activated currents present in Deiters' cells. Hear Res. 1998; 123:168–182. PMID: 9745965.
Article11. Szucs A, Somodi S, Batta TJ, Tóth A, Szigeti GP, Csernoch L, Panyi G, Sziklai I. Differential expression of potassium currents in Deiters' cells of the guinea pig cochlea. Pflugers Arch. 2006; 452:332–341. PMID: 16447073.12. Qu C, Liang F, Hu W, Shen Z, Spicer SS, Schulte BA. Expression of CLC-K chloride channels in the rat cochlea. Hear Res. 2006; 213:79–87. PMID: 16466872.
Article13. Castle NA. Selective inhibition of potassium currents in rat ventricle by clofilium and its tertiary homolog. J Pharmacol Exp Ther. 1991; 257:342–350. PMID: 2019997.14. Steidl JV, Yool AJ. Distinct mechanisms of block of Kv1.5 channels by tertiary and quaternary amine clofilium compounds. Biophys J. 2001; 81:2606–2613. PMID: 11606274.
Article15. Yeola SW, Snyders DJ. Electrophysiological and pharmacological correspondence between Kv4.2 current and rat cardiac transient outward current. Cardiovasc Res. 1997; 33:540–547. PMID: 9093524.
Article16. Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996; 79:659–668. PMID: 8831489.17. Rudy B, Kentros C, Vela-Saenz De Miera E. Families of potassium channel genes in mammals: Toward an understanding of the molecular basis of potassium channel diversity. Mol Cell Neurosci. 1991; 2:89–102. PMID: 19912787.
Article18. Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M. Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol Rev. 2000; 52:557–594. PMID: 11121510.19. Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002; 165:1–9. PMID: 12031509.20. Marcus DC, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol. 2002; 282:C403–C407. PMID: 11788352.
Article21. Mori N, Sakagami M, Fukazawa K, Matsunaga T. An immunohistochemical and electrophysiological study on Isk protein in the stria vascularis of the guinea pig. Eur Arch Otorhinolaryngol. 1993; 250:186–189. PMID: 8357611.
Article22. Rossi ML, Ferrary E, Martini A, Martini M, Pelucchi B, Bernard C, Teixeira M, Sterkers O, Fesce R. The effect of clofilium, a K-channel blocker, on the electrogenic K secretion and the sensory discharge at the frog semicircular canal. Brain Res. 1996; 721:174–180. PMID: 8793098.
Article23. Lagostena L, Cicuttin A, Inda J, Kachar B, Mammano F. Frequency dependence of electrical coupling in Deiters' cells of the guinea pig cochlea. Cell Commun Adhes. 2001; 8:393–399. PMID: 12064625.
Article24. Yang J, Wang J. Possible function of outward potassium currents in isolated Deiters' cells of guinea pig cochlea. Chin Med J (Engl). 2002; 115:264–267. PMID: 11940345.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Outward Rectifying Current in Isolated Deiters' Cells from Guinea Pig Cochlea
- Temperature Enhances Activation and Inactivation Kinetics of Potassium Currents in Inner Hair Cells Isolated from Guinea-Pig Cochlea
- Modulation of outward potassium currents by nitric oxide in longitudinal smooth muscle cells of guinea-pig ileum
- Effects of Aminoglycoside Antibiotics on Acetylcholine-induced Potassium Currents in Guinea-pig Outer Hair Cell
- The Effects of Neurotransmitters on the Ion Channels in the Isolated Deiters'Cells of Guinea Pig Cochlea