Korean J Physiol Pharmacol.
2009 Dec;13(6):417-424. 10.4196/kjpp.2009.13.6.417.
Hexane-Soluble Fraction of the Common Fig, Ficus carica, Inhibits Osteoclast Differentiation in Murine Bone Marrow-Derived Macrophages and RAW 264.7 Cells
- Affiliations
-
- 1Department of Dental Pharmacology, School of Dentistry, and Institute of Oral Bioscience, Brain Korea 21 Project, Chonbuk National University, Jeon-Ju 561-756, Korea. ysoh@jbnu.ac.kr
- 2College of Pharmacy, Woosuk University, Sam-Rye 565-701, Korea.
- 3Department of Pathology, School of Medicine, Chonbuk National University, Jeon-Ju 561-756, Korea.
- 4Department of Oral Pathology, School of Dentistry, Chonbuk National University, Jeon-Ju 561-756, Korea.
- 5Department of Dental Biomaterials, School of Dentistry, Chonbuk National University, Jeon-Ju 561-756, Korea.
- 6Department of Materials Science and Metallurgical Engineering, Sunchon National University, Sunchon 540-742, Korea.
- KMID: 2285375
- DOI: http://doi.org/10.4196/kjpp.2009.13.6.417
Abstract
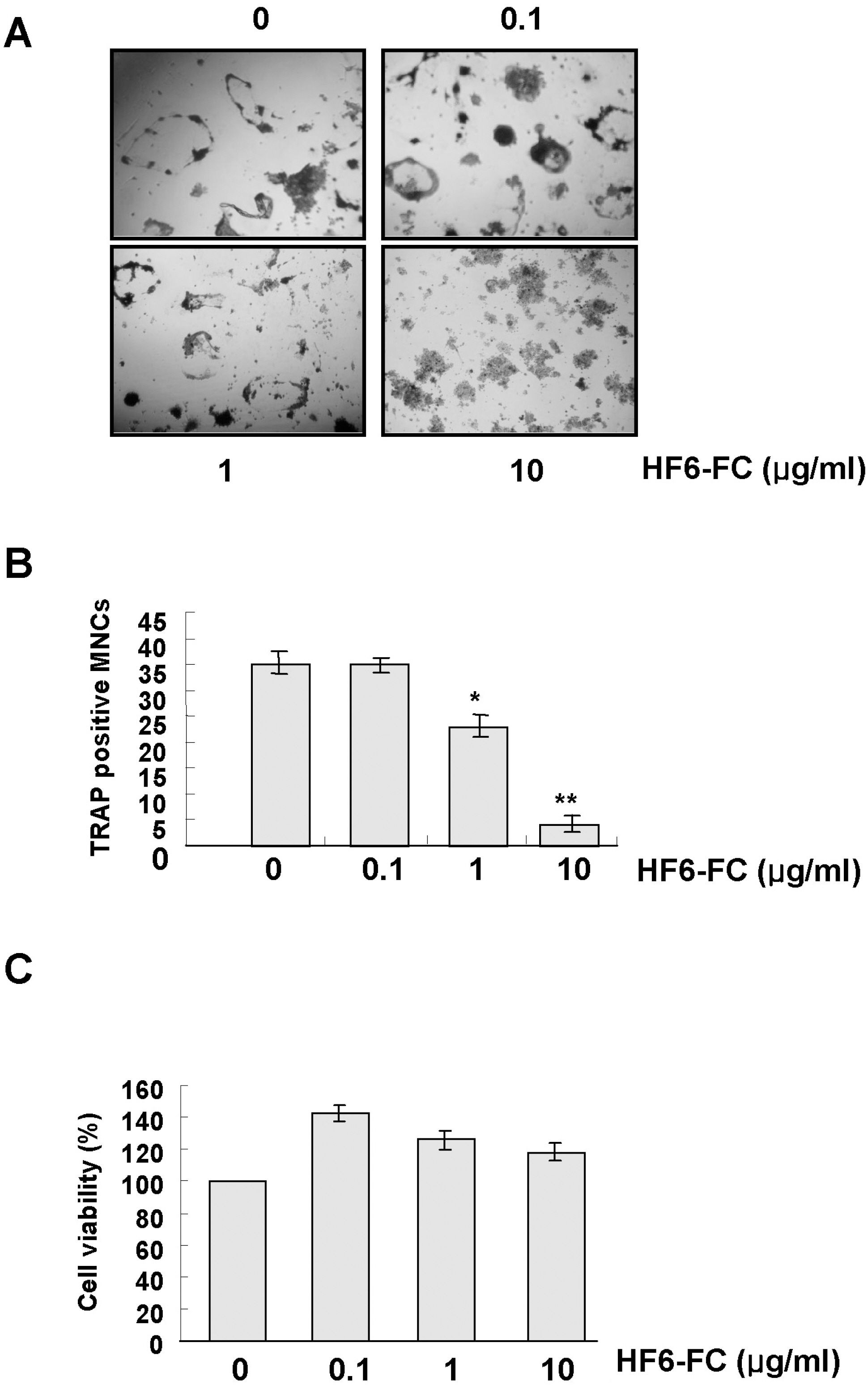
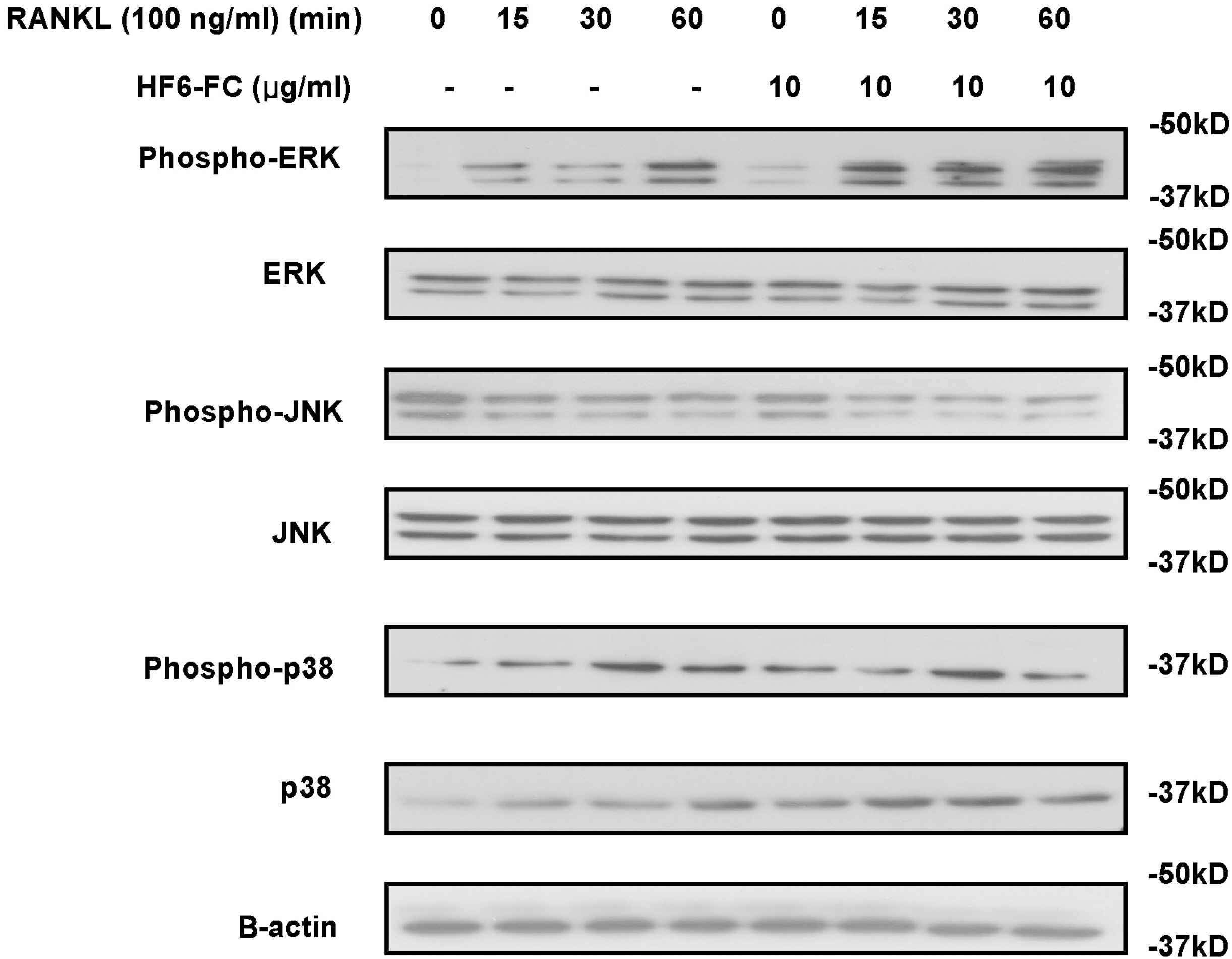
- Osteoclasts, derived from multipotent myeloid progenitor cells, play homeostatic roles in skeletal modeling and remodeling, but may also destroy bone in pathological conditions such as osteoporosis and rheumatoid arthritis. Osteoclast development depends critically on a differentiation factor, the receptor activator of NF-kappaB ligand (RANKL). In this study, we found that the hexane soluble fraction of the common fig Ficus carica (HF6-FC) is a potent inhibitor of osteoclastogenesis in RANKL-stimulated RAW264.7 cells and in bone marrow-derived macrophages (BMMs). HF6-FC exerts its inhibitory effects by suppression of p38 and NF-kappaB but activation of ERK. In addition, HF6-FC significantly decreased the expression of NFATc1 and c-Fos, the master regulator of osteoclast differentiation. The data indicate that components of HF6-FC may have therapeutic effects on bone-destructive processes such as osteoporosis, rheumatoid arthritis, and periodontal bone resorption.
Keyword
MeSH Terms
Figure
Reference
-
References
Ahmed W., Khan AQ., Malik A. Two Triterpenes from the Leaves of Ficus carica. Planta Med. 54:481. 1988.Alliston T., Derynck R. Medicine: interfering with bone remodelling. Nature. 416:686–687. 2002.Canal JR., Torres MD., Romero A., Pérez C. A chloroform extract obtained from a decoction of Ficus carica leaves improves the cholesterolaemic status of rats with streptootocin-includede diabetes. Acta Physiol Hung. 87:71–76. 2000.
ArticleChang EJ., Kim HJ., Ha J., Kim HJ., Ryu J., Park KH., Kim UH., Lee ZH., Kim HM., Fisher DE., Kim HH. Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J Cell Sci. 120:166–176. 2007.
ArticleChoi HJ., Song BJ., Gong YD., Gwak WJ., Soh Y. Rapid degradation of hypoxia-inducible factor-1alpha by KRH102053, a new activator of prolyl hydroxylase 2. Br J Pharmacol. 154:114–125. 2008.Coon D., Gulati A., Cowan C., He J. The role of cyclooxygenase-2 (COX-2) in inflammatory bone resorption. J Endod. 33:432–436. 2007.
ArticleEl-Kholy IS., Shaban MA. Constituents of the leaves of Ficus carica, L. II. Isolation of a psi-taraxasteryl ester, rutin, and a new steroid sapogenin. J Chem Soc Perkin. 13:1140–1142. 1966.Han KY., Yang D., Chang EJ., Lee Y., Huang H., Sung SH., Lee ZH., Kim YC., Kim HH. Inhibition of osteoclast differentiation and bone resorption by sauchinone. Biochem Pharmacol. 74:911–923. 2007.
ArticleJimi E., Aoki K., Saito H., D'Acquisto F., May MJ., Nakamura I., Sudo T., Kojima T., Okamoto F., Fukushima H., Okabe K., Ohya K., Ghosh S. Selective in hibition of NF-kappa B block osteoclastogenesis and prevents inflammatory bone destructin in vivo. Nat Med. 6:617–624. 2004.Kobayashi N., Kadono Y., Naito A., Matsumoto K., Yamamoto T., Tanaka S., Inoue J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO. 20:1271–1280. 2001.
ArticleLee SE., Woo KM., Kim SY., Kim HM., Kwack K., Lee ZH., Kim HH. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 30:71–77. 2002.
ArticleLee WT. Coloured standard illustrations of Korean plants. Academy press, Seoul, p 624. 1996.Luo JL., Kamata H., Karin M. IKK/NF-kappaB signaling: balancing life and death–a new approach to cancer therapy. J Clin Invest. 115:2625–2632. 2005.Mino T., Sugiyama E., Taki H., Kuroda A., Yamashita N., Maruyama M., Kobayashi M. Interleukin-1alpha and tumor necrosis factor alpha synergistically stimulate prostaglandin E2-dependent production of interleukin-11 in rheumatoid synovial fibroblasts. Arthritis Rheum. 41:2004–2013. 1998.Miyaura C., Inada M., Matsumoto C., Ohshiba T., Uozumi N., Shimizu T., Ito A. An essential role of cytosolic phospholipase A2alpha in prostaglandin E2-mediated bone resorption associated with inflammation. J Exp Med. 197:1303–1310. 2003.Miyazaki T., Katagiri H., Kanegae Y., Takayanagi H., Sawada Y., Yamamoto A., Pando MP., Asano T., Verma IM., Oda H., Nakamura K., Tanaka S. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J Cell Biol. 148:333–342. 2000.Motyckova G., Weilbaecher KN., Horstmann M., Rieman DJ., Fisher DZ., Fisher DE. Linking osteopetrosis and pycnodysostosis: regulation of cathepsin K expression by the microphthalmia transcription factor family. Proc Natl Acad Sci U S A. 98:5798–5803. 2001.
ArticleMozar A., Haren N., Chasseraud M., Louvet L., Maziere C., Wattel A., Mentaverri R., Morliere P., Kamel S., Brazier M., Maziere JC., Massy ZA. High extracellular inorganic phosphate concentration inhibits RANK-RANKL signaling in osteoclast-like cells. J Cell Physiol. 215:47–54. 2008.
ArticleOh SY., Aryal DK., Kim YG., Kim HG. Effects of R. Glutinosa and E. Senticosus on Postmenopausal Osteoporosis. Korean J Physiol Pharmacol. 11:121–127. 2007.Reddy SV., Hundley JE., Windle JJ., Alcantara O., Linn R., Leach RJ., Boldt DH., Roodman GD. Characterization of the mouse tartrate-resistant acid phosphatase (TRAP) gene promoter. J Bone Miner Res. 10:601–606. 1995.
ArticleRubnov S., Kashman Y., Rabinowitz R., Schlesinger M., Mechoulam R. Suppressors of cancer cell proliferation from Fig (Ficus carica) Resin: isolation and structure elucidation. J Nat Prod. 64:993–996. 2001.
ArticleSerraclara A., Hawkins F., Pérez C., Domínguez E., Campillo JE., Torres MD. Hypoglycemic action of an oral fig-leaf decoction in type-I diabetic patients. Diabetes Res Clin Pract. 39:19–22. 1998.
ArticleSoh Y., Jeong KS., Lee IJ., Bae MA., Kim YC., Song BJ. Selective activation of the c-Jun N-terminal protein kinase pathway during 4-hydroxynonenal-induced apoptosis of PC12 cells. Mol Pharmacol. 58:534–541. 2000.
ArticleSoh Y., Shin MH., Lee JS., Jang JH., Kim OH., Kang H., Surh YJ. Oxidative DNA damage and glioma cell death induced by tetrahydropapaveroline. Mutat Res. 544:129–142. 2003.
ArticleStern PH. Antiresorptive agents and osteoclast apoptosis. J Cell Biochem. 101:1087–1096. 2007.
ArticleTeitelbaum SL., Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 4:638–649. 2003.
ArticleTheill LE., Boyle WJ., Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 20:795–823. 2002.
ArticleVaira S., Alhawagri M., Anwisye I., Kitaura H., Faccio R., Novack DV. RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice. J Clin Invest. 118:2088–2097. 2008.
ArticleWei S., Kitaura H., Zhou P., Ross FP., Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 115:282–290. 2005.
ArticleWong BR., Besser D., Kim N., Arron JR., Vologodskaia M., Hanafusa H., Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 4:1041–1049. 1999.
ArticleWong BR., Josien R., Lee SY., Vologodskaia M., Steinman RM., Choi Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J Biol Chem. 273:28355–28359. 1998.Yamashita T., Yao Z., Li F., Zhang Q., Badell IR., Schwarz EM., Takeshita S., Wagner EF., Noda M., Matsuo K., Xing L., Boyce BF. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 282:18245–18253. 2007.Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz LD., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 345:442–444. 1990.
ArticleZhou B., Cron RQ., Wu B., Genin A., Wang Z., Liu S., Robson P., Baldwin HS. Regulation of the murine Nfatc1 gene by NFATc2. J Biol Chem. 277:10704–10711. 2002.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NDRG2 Expression Decreases Tumor-Induced Osteoclast Differentiation by Down-regulating ICAM1 in Breast Cancer Cells
- Antimicrobial Activity of Methanol Extract from Ficus carica Leaves Against Oral Bacteria
- Anti-inflammatory Activity and Modulation of Endoplasmic Reticulum Stress by the Hexane Fraction from the Roots of Peucedanum insolens Kitag
- Induction of apoptosis by methanol extracts of Ficus carica L. in FaDu human hypopharynx squamous carcinoma cells
- Stimulation of macrophage function by S-antigen: production of nitric oxide