Intest Res.
2015 Apr;13(2):175-179. 10.5217/ir.2015.13.2.175.
Natural Course of an Untreated Metastatic Perirectal Lymph Node After the Endoscopic Resection of a Rectal Neuroendocrine Tumor
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. dhyang@amc.seoul.kr
- 3Department of Colon and Rectal Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2284886
- DOI: http://doi.org/10.5217/ir.2015.13.2.175
Abstract
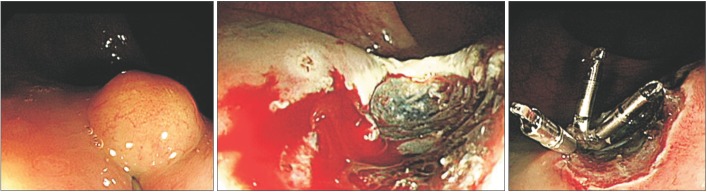
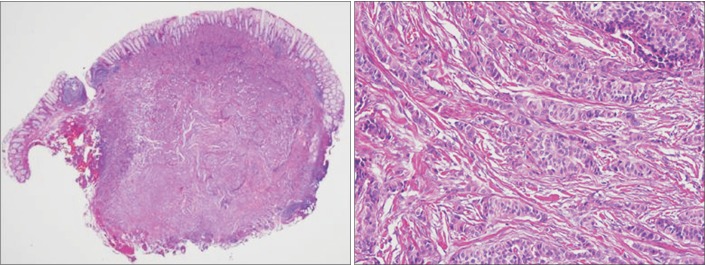
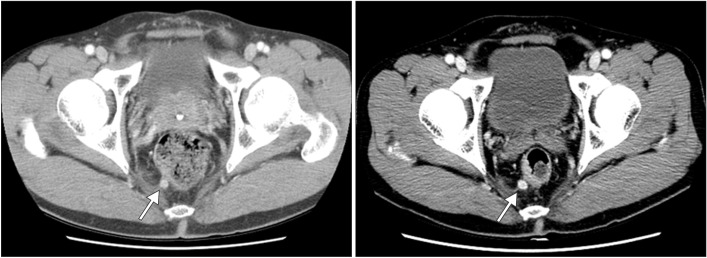
- Lymph node metastasis is rare in small (i.e., <10 mm) rectal neuroendocrine tumors (NETs). In addition to tumor size, pathological features such as the mitotic or Ki-67 proliferation index are associated with lymph node metastasis in rectal NETs. We recently treated a patient who underwent endoscopic treatment of a small, grade 1 rectal NET that recurred in the form of perirectal lymph node metastasis 7 years later. A 7-mm-sized perirectal lymph node was noted at the time of the initial endoscopic treatment. The same lymph node was found to be slightly enlarged on follow-up and finally confirmed as a metastatic NET. Therefore, the perirectal lymph node metastasis might have been present at the time of the initial diagnosis. However, the growth rate of the lymph node was extremely low, and it took 7 years to increase in size from 7 to 10 mm. NETs with low Ki-67 proliferation index and without mitotic activity may grow extremely slowly even if they are metastatic.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Are Small Rectal Neuroendocrine Tumors Safe?
Jae Ho Choi, Jae Myung Cha
Intest Res. 2015;13(2):103-104. doi: 10.5217/ir.2015.13.2.103.Usefulness of endoscopic resection using the band ligation method for rectal neuroendocrine tumors
Ju Seung Kim, Yoon Jae Kim, Jun-Won Chung, Jung Ho Kim, Kyoung Oh Kim, Kwang An Kwon, Dong Kyun Park, Jung Suk An
Intest Res. 2016;14(2):164-171. doi: 10.5217/ir.2016.14.2.164.
Reference
-
1. Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011; 40:1–18. PMID: 21349409.2. Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010; 105:2563–2569. PMID: 20823835.3. Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in Taiwan: a nation-wide cancer registry-based study. PLoS One. doi:10.1371/journal.pone.0062487. Published online 22 April 2013.4. Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010; 45:234–243. PMID: 20058030.5. Scherubl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy. 2009; 41:162–165. PMID: 19214898.6. Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg. 1994; 179:231–248. PMID: 8044398.7. Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005; 103:1587–1595. PMID: 15742328.8. Weinstock B, Ward SC, Harpaz N, Warner RR, Itzkowitz S, Kim MK. Clinical and prognostic features of rectal neuroendocrine tumors. Neuroendocrinology. 2013; 98:180–187. PMID: 24080744.9. Jernman J, Valimaki MJ, Louhimo J, Haglund C, Arola J. The novel WHO 2010 classification for gastrointestinal neuroendocrine tumours correlates well with the metastatic potential of rectal neuroendocrine tumours. Neuroendocrinology. 2012; 95:317–324. PMID: 22327359.10. Hotta K, Shimoda T, Nakanishi Y, Saito D. Usefulness of Ki-67 for predicting the metastatic potential of rectal carcinoids. Pathol Int. 2006; 56:591–596. PMID: 16984615.11. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press;2010.12. Lee JL, Yu CS, Kim M, Hong SM, Lim SB, Kim JC. Prognostic impact of diagnosing colorectal neuroendocrine carcinoma using the World Health Organization 2010 classification. Surgery. 2014; 155:650–658. PMID: 24468036.13. Kim GU, Kim KJ, Hong SM, et al. Clinical outcomes of rectal neuroendocrine tumors ≤ 10 mm following endoscopic resection. Endoscopy. 2013; 45:1018–1023. PMID: 24288222.14. Kim DH, Lee JH, Cha YJ, et al. Surveillance strategy for rectal neuroendocrine tumors according to recurrence risk stratification. Dig Dis Sci. 2014; 59:850–856. PMID: 24323182.15. Kim MS, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Clinical outcomes for rectal carcinoid tumors according to a new (AJCC 7th edition) TNM staging system: a single institutional analysis of 122 patients. J Surg Oncol. 2013; 107:835–841. PMID: 23505038.16. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging-a meta-analysis. Radiology. 2004; 232:773–783. PMID: 15273331.17. Mandair D, Caplin ME. Colonic and rectal NET's. Best Pract Res Clin Gastroenterol. 2012; 26:775–789. PMID: 23582918.18. Adams S, Baum R, Rink T, Schumm-Drager PM, Usadel KH, Hor G. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med. 1998; 25:79–83. PMID: 9396878.19. Sasaki Y, Niwa Y, Hirooka Y, et al. The use of endoscopic ultrasound-guided fine-needle aspiration for investigation of submucosal and extrinsic masses of the colon and rectum. Endoscopy. 2005; 37:154–160. PMID: 15692931.20. Bakker IS, Snijders HS, Wouters MW, et al. High complication rate after low anterior resection for mid and high rectal cancer; results of a population-based study. Eur J Surg Oncol. 2014; 40:692–698. PMID: 24655803.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A small, low-grade rectal neuroendocrine tumor with lateral pelvic lymph node metastasis: a case report
- Endoscopic Treatment Outcome of Rectal Neuroendocrine Tumors Removed by Ligation-assisted Endoscopic Submucosal Resection
- Role of CT in evaluating rectal cancer: on the aspect of perirectal fat infiltration and lymph node involvement
- Endoscopic treatment for rectal neuroendocrine tumor: which method is better?
- Recurrence after endoscopic resection of small rectal neuroendocrine tumors: a retrospective cohort study