Diabetes Metab J.
2014 Jun;38(3):163-170. 10.4093/dmj.2014.38.3.163.
Interrelationships between the Retinal Neuroglia and Vasculature in Diabetes
- Affiliations
-
- 1Department of Medicine, Case Western Reserve University Medicine, Cleveland, OH, USA. tsk@case.edu.
- 2Department of Medicine, Louis Stokes Cleveland Veterans Administration Medical Center, Cleveland, OH, USA.
- KMID: 2280685
- DOI: http://doi.org/10.4093/dmj.2014.38.3.163
Abstract
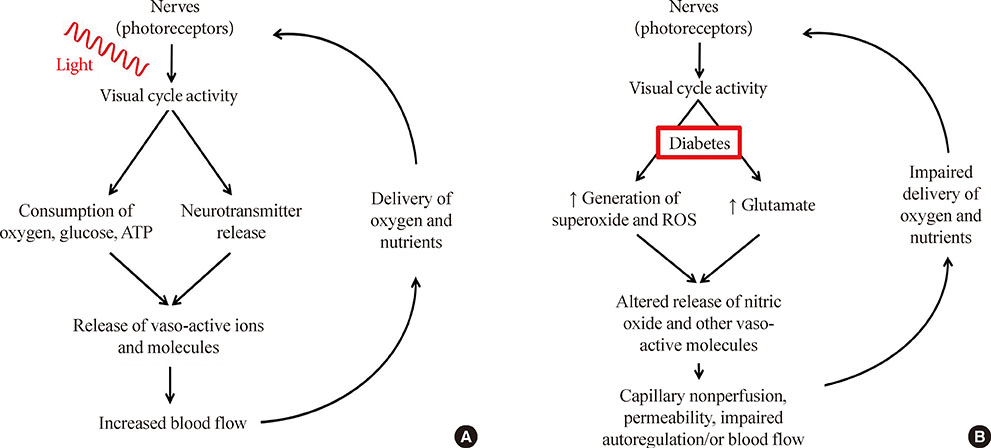
- For years, diabetic retinopathy has been defined based on vascular lesions, and neural abnormalities were not regarded as important. This review summarizes evidence that the neural retina has important effects on the retinal vasculature under normal conditions, and the interaction between the retinal neuroglial cells and vascular function is altered in diabetes. Importantly, new evidence raises a possibility that abnormalities within retinal neuroglial cells (notably photoreceptors) might actually be causing or initiating the vascular disease in diabetic retinopathy.
MeSH Terms
Figure
Reference
-
1. Buerk DG, Riva CE, Cranstoun SD. Frequency and luminance-dependent blood flow and K+ ion changes during flicker stimuli in cat optic nerve head. Invest Ophthalmol Vis Sci. 1995; 36:2216–2227.2. Falsini B, Riva CE, Logean E. Flicker-evoked changes in human optic nerve blood flow: relationship with retinal neural activity. Invest Ophthalmol Vis Sci. 2002; 43:2309–2316.3. Logean E, Falsini B, Riva CE. Effect of chromatic flicker on circulation of the optic nerve. Klin Monbl Augenheilkd. 2001; 218:345–347.4. Riva CE, Logean E, Falsini B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog Retin Eye Res. 2005; 24:183–215.5. Hardarson SH, Basit S, Jonsdottir TE, Eysteinsson T, Halldorsson GH, Karlsson RA, Beach JM, Benediktsson JA, Stefansson E. Oxygen saturation in human retinal vessels is higher in dark than in light. Invest Ophthalmol Vis Sci. 2009; 50:2308–2311.6. Havelius U, Hansen F. Ocular vasodynamic changes in light and darkness in smokers. Invest Ophthalmol Vis Sci. 2005; 46:1698–1705.7. Formaz F, Riva CE, Geiser M. Diffuse luminance flicker increases retinal vessel diameter in humans. Curr Eye Res. 1997; 16:1252–1257.8. Riva CE, Harino S, Shonat RD, Petrig BL. Flicker evoked increase in optic nerve head blood flow in anesthetized cats. Neurosci Lett. 1991; 128:291–296.9. Scheiner AJ, Riva CE, Kazahaya K, Petrig BL. Effect of flicker on macular blood flow assessed by the blue field simulation technique. Invest Ophthalmol Vis Sci. 1994; 35:3436–3441.10. Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010; 2:pii5.11. Bill A, Sperber GO. Aspects of oxygen and glucose consumption in the retina: effects of high intraocular pressure and light. Graefes Arch Clin Exp Ophthalmol. 1990; 228:124–127.12. Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab. 2005; 25:2–16.13. Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007; 10:1369–1376.14. Jakovcevic D, Harder DR. Role of astrocytes in matching blood flow to neuronal activity. Curr Top Dev Biol. 2007; 79:75–97.15. Schmetterer L, Polak K. Role of nitric oxide in the control of ocular blood flow. Prog Retin Eye Res. 2001; 20:823–847.16. Toda N, Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007; 26:205–238.17. Kondo M, Wang L, Bill A. The role of nitric oxide in hyperaemic response to flicker in the retina and optic nerve in cats. Acta Ophthalmol Scand. 1997; 75:232–235.18. Metea MR, Newman EA. Signalling within the neurovascular unit in the mammalian retina. Exp Physiol. 2007; 92:635–640.19. Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nat Rev Neurosci. 2005; 6:77–85.20. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003; 9:669–676.21. Ruprecht K, Stadelmann C, Hummel V, Klein O, Bruck W, Rieckmann P. Brain derived neurotrophic factor does not act on adult human cerebral endothelial cells. Neurosci Lett. 2002; 330:175–178.22. Luu CD, Szental JA, Lee SY, Lavanya R, Wong TY. Correlation between retinal oscillatory potentials and retinal vascular caliber in type 2 diabetes. Invest Ophthalmol Vis Sci. 2010; 51:482–486.23. Garhöfer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004; 88:887–891.24. Dorner GT, Garhofer G, Huemer KH, Riva CE, Wolzt M, Schmetterer L. Hyperglycemia affects flicker-induced vasodilation in the retina of healthy subjects. Vision Res. 2003; 43:1495–1500.25. Lecleire-Collet A, Audo I, Aout M, Girmens JF, Sofroni R, Erginay A, Le Gargasson JF, Mohand-Said S, Meas T, Guillausseau PJ, Vicaut E, Paques M, Massin P. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2011; 52:2861–2867.26. Nguyen TT, Kawasaki R, Wang JJ, Kreis AJ, Shaw J, Vilser W, Wong TY. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care. 2009; 32:2075–2080.27. Mandecka A, Dawczynski J, Vilser W, Blum M, Muller N, Kloos C, Wolf G, Muller UA. Abnormal retinal autoregulation is detected by provoked stimulation with flicker light in well-controlled patients with type 1 diabetes without retinopathy. Diabetes Res Clin Pract. 2009; 86:51–55.28. Mandecka A, Dawczynski J, Blum M, Muller N, Kloos C, Wolf G, Vilser W, Hoyer H, Muller UA. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care. 2007; 30:3048–3052.29. Nguyen TT, Kawasaki R, Kreis AJ, Wang JJ, Shaw J, Vilser W, Wong TY. Correlation of light-flicker-induced retinal vasodilation and retinal vascular caliber measurements in diabetes. Invest Ophthalmol Vis Sci. 2009; 50:5609–5613.30. Mishra A, Newman EA. Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia. 2010; 58:1996–2004.31. Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998; 102:783–791.32. Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004; 45:3330–3336.33. Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008; 586(Pt 18):4401–4408.34. Palmowski AM, Sutter EE, Bearse MA Jr, Fung W. Mapping of retinal function in diabetic retinopathy using the multifocal electroretinogram. Invest Ophthalmol Vis Sci. 1997; 38:2586–2596.35. Lieth E, Gardner TW, Barber AJ, Antonetti DA. Penn State Retina Research Group. Retinal neurodegeneration: early pathology in diabetes. Clin Experiment Ophthalmol. 2000; 28:3–8.36. Jackson GR, Scott IU, Quillen DA, Walter LE, Gardner TW. Inner retinal visual dysfunction is a sensitive marker of non-proliferative diabetic retinopathy. Br J Ophthalmol. 2012; 96:699–703.37. Trick GL, Burde RM, Gordon MO, Santiago JV, Kilo C. The relationship between hue discrimination and contrast sensitivity deficits in patients with diabetes mellitus. Ophthalmology. 1988; 95:693–698.38. Masha A, Dinatale S, Allasia S, Martina V. Role of the decreased nitric oxide bioavailability in the vascular complications of diabetes mellitus. Curr Pharm Biotechnol. 2011; 12:1354–1363.39. Kowluru RA, Engerman RL, Case GL, Kern TS. Retinal glutamate in diabetes and effect of antioxidants. Neurochem Int. 2001; 38:385–390.40. Obrosova IG, Drel VR, Kumagai AK, Szabo C, Pacher P, Stevens MJ. Early diabetes-induced biochemical changes in the retina: comparison of rat and mouse models. Diabetologia. 2006; 49:2525–2533.41. Deng J, Wu DZ, Gao R. Detection of glutamate and gamma-aminobutyric acid in vitreous of patients with proliferative diabetic retinopathy. Yan Ke Xue Bao. 2000; 16:199–202.42. Ambati J, Chalam KV, Chawla DK, D'Angio CT, Guillet EG, Rose SJ, Vanderlinde RE, Ambati BK. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1997; 115:1161–1166.43. Schlingemann RO, Witmer AN. Treatment of retinal diseases with VEGF antagonists. Prog Brain Res. 2009; 175:253–267.44. Jardeleza MS, Miller JW. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol. 2009; 24:87–92.45. Yang Z, Mo X, Gong Q, Pan Q, Yang X, Cai W, Li C, Ma JX, He Y, Gao G. Critical effect of VEGF in the process of endothelial cell apoptosis induced by high glucose. Apoptosis. 2008; 13:1331–1343.46. Bai Y, Ma JX, Guo J, Wang J, Zhu M, Chen Y, Le YZ. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009; 219:446–454.47. Hammes HP, Federoff HJ, Brownlee M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med. 1995; 1:527–534.48. Steinle JJ, Granger HJ. Nerve growth factor regulates human choroidal, but not retinal, endothelial cell migration and proliferation. Auton Neurosci. 2003; 108:57–62.49. Elshaer SL, Abdelsaid MA, Al-Azayzih A, Kumar P, Matragoon S, Nussbaum JJ, El-Remessy AB. Pronerve growth factor induces angiogenesis via activation of TrkA: possible role in proliferative diabetic retinopathy. J Diabetes Res. 2013; 2013:432659.50. de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, Stitt AW. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci. 2006; 47:5561–5568.51. Arden GB. The absence of diabetic retinopathy in patients with retinitis pigmentosa: implications for pathophysiology and possible treatment. Br J Ophthalmol. 2001; 85:366–370.52. Arden GB, Gunduz MK, Kurtenbach A, Volker M, Zrenner E, Gunduz SB, Kamis U, Ozturk BT, Okudan S. A preliminary trial to determine whether prevention of dark adaptation afthe course of early diabetic retinopathy. Eye (Lond). 2010; 24:1149–1155.53. Arden GB, Jyothi S, Hogg CH, Lee YF, Sivaprasad S. Regression of early diabetic macular oedema is associated with prevention of dark adaptation. Eye (Lond). 2011; 25:1546–1554.54. Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci U S A. 2013; 110:16586–16591.55. Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011; 30:343–358.56. Zheng L, Kern TS. Role of nitric oxide, superoxide, peroxynitrite and poly(ADP-ribose) polymerase in diabetic retinopathy. Front Biosci (Landmark Ed). 2009; 14:3974–3987.57. Tang J, Du Y, Lee CA, Talahalli R, Eells JT, Kern TS. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro. Invest Ophthalmol Vis Sci. 2013; 54:3681–3690.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Electron Microscopic Features of Epiretinal Membrane in Rhegmatogenous Retinal Detachment
- Neuroglial Cell and Alzheimer's Disease
- Association between Retinal Vascular Geometric Changes and Cognitive Impairment: A Systematic Review and Meta-Analysis
- Morphologic and Immunohistochemical Studies of Attached Retina in Intravitreal Silicone Oil
- A Case Report of Bilateral Retinal Racemose Hemangioma Restricted to Peripapillary Area