Blood Res.
2014 Dec;49(4):265-269. 10.5045/br.2014.49.4.265.
Progress of in vitro factor VIII coagulant activity from 0 to 8 hours after reconstitution
- Affiliations
-
- 1Department of Pediatrics, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
- 2Department of Pediatrics, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea.
- 3Department of Pediatrics, Incheon Medical Center Beakryung Hospital, Incheon, Korea.
- 4Division of Pediatric Hematology/Oncology, Asan Medical Center Children's Hospital, Department of Pediatrics, University of Ulsan College of Medicine, Seoul, Korea.
- 5Green Cross Laboratories, Yongin, Korea.
- 6Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. drhyun@yonsei.ac.kr
- KMID: 2270685
- DOI: http://doi.org/10.5045/br.2014.49.4.265
Abstract
- BACKGROUND
Continuous infusion of factor VIII (FVIII) is a more cost-effective method for treating hemophilia A than intermittent bolus injection. However, there is currently no specific data in Korea about the progress of in vitro FVIII coagulant activity (FVIII:C) after reconstitution from its lyophilized form.
METHODS
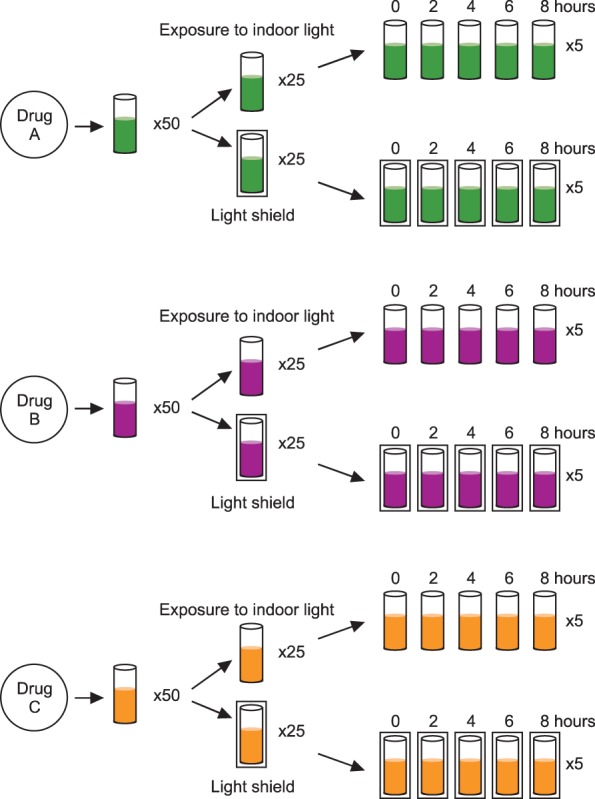
Three commercial FVIII concentrate products (two recombinant FVIII and one plasma-derived) were used. In vitro FVIII:C was measured at 0, 2, 4, 6, and 8 hours following reconstitution in both the indoor light-exposed and light-shielded groups.
RESULTS
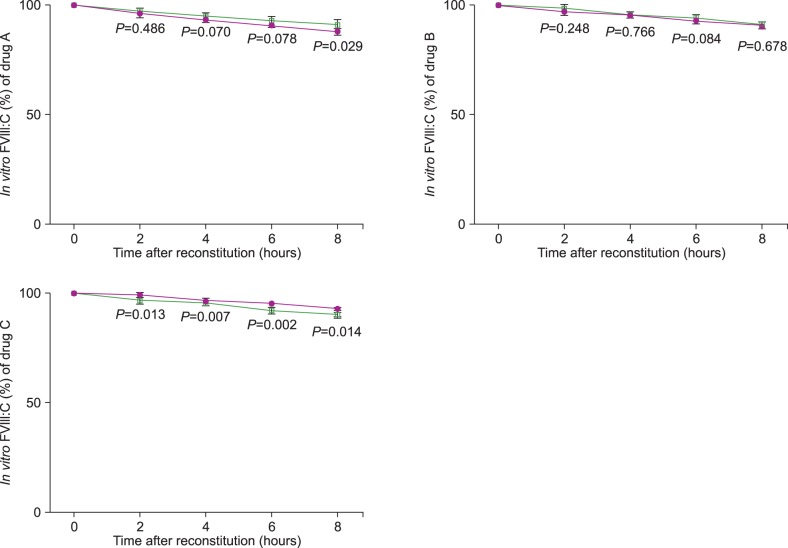
For the three drugs, in vitro FVIII:C decreased over the 8 hours following reconstitution (P<0.001). The decline of FVIII:C was linear (P<0.001). In vitro FVIII:C for the indoor light-exposed groups was 95.3+/-1.9% and 90.6+/-2.5% after 4 and 8 hours following reconstitution, respectively, compared to baseline activity. In the light-shielded group, FVIII:C was 95.4+/-1.1% and 90.9+/-1.7% of the baseline activity after 4 and 8 hours, respectively. There was no statistical difference between FVIII:C in the indoor light-exposed and light-shielded groups (P=0.849).
CONCLUSION
In vitro FVIII:C decreased after reconstitution, but activity was maintained at over 90% of the baseline value during 8 hours. Exposure to indoor light did not accelerate the loss of FVIII:C over the experimental time. This result indicates that CI with FVIII is available in 8-hour intervals, with no indoor light-exposure precautions needed.
Keyword
MeSH Terms
Figure
Reference
-
1. Batorova A, Martinowitz U. Continuous infusion of coagulation factors: current opinion. Curr Opin Hematol. 2006; 13:308–315. PMID: 16888434.
Article3. Batorova A, Martinowitz U. Intermittent injections vs. continuous infusion of factor VIII in haemophilia patients undergoing major surgery. Br J Haematol. 2000; 110:715–720. PMID: 10997985.
Article4. Hathaway WE, Christian MJ, Clarke SL, Hasiba U. Comparison of continuous and intermittent Factor VIII concentrate therapy in hemophilia A. Am J Hematol. 1984; 17:85–88. PMID: 6430068.
Article5. Bidlingmaier C, Deml MM, Kurnik K. Continuous infusion of factor concentrates in children with haemophilia A in comparison with bolus injections. Haemophilia. 2006; 12:212–217. PMID: 16643203.
Article6. Batorova A, Holme P, Gringeri A, et al. Continuous infusion in haemophilia: current practice in Europe. Haemophilia. 2012; 18:753–759. PMID: 22530687.
Article7. Auerswald G, Bade A, Haubold K, Overberg D, Masurat S, Moorthi C. No inhibitor development after continuous infusion of factor concentrates in subjects with bleeding disorders undergoing surgery: a prospective study. Haemophilia. 2013; 19:438–444. PMID: 23279056.
Article8. Berntorp E, Bjorkman S. The pharmacokinetics of clotting factor therapy. Haemophilia. 2003; 9:353–359. PMID: 12828668.
Article9. Shapiro AD, Korth-Bradley J, Poon MC. Use of pharmacokinetics in the coagulation factor treatment of patients with haemophilia. Haemophilia. 2005; 11:571–582. PMID: 16236106.
Article10. Aronstam A, McLellan DS, Wassef M, Mbatha PS. Effect of height and weight on the in vivo recovery of transfused factor VIII C. J Clin Pathol. 1982; 35:289–291. PMID: 6802879.
Article11. Fukui H, Yoshioka A, Shima M, et al. Clinical evaluation of recombinant human factor VIII (BAY w 6240) in the treatment of hemophilia A. Int J Hematol. 1991; 54:419–427. PMID: 1756252.12. Morfini M, Longo G, Messori A, Lee M, White G, Mannucci P. The Recombinate Study Group. Pharmacokinetic properties of recombinant factor VIII compared with a monoclonally purified concentrate (Hemofil M). Thromb Haemost. 1992; 68:433–435. PMID: 1448776.
Article13. Fijnvandraat K, Peters M, ten Cate JW. Inter-individual variation in half-life of infused recombinant factor VIII is related to pre-infusion von Willebrand factor antigen levels. Br J Haematol. 1995; 91:474–476. PMID: 8547097.
Article14. Fijnvandraat K, Berntorp E, ten Cate JW, et al. Recombinant, B-domain deleted factor VIII (r-VIII SQ): pharmacokinetics and initial safety aspects in hemophilia A patients. Thromb Haemost. 1997; 77:298–302. PMID: 9157585.
Article15. White GC 2nd, Courter S, Bray GL, Lee M, Gomperts ED. The Recombinate Previously Treated Patient Study Group. A multicenter study of recombinant factor VIII (Recombinate) in previously treated patients with hemophilia A. Thromb Haemost. 1997; 77:660–667. PMID: 9134639.16. Vlot AJ, Mauser-Bunschoten EP, Zarkova AG, et al. The half-life of infused factor VIII is shorter in hemophiliac patients with blood group O than in those with blood group A. Thromb Haemost. 2000; 83:65–69. PMID: 10669157.17. Mondorf W, Klinge J, Luban NL, et al. Low factor VIII recovery in haemophilia A patients without inhibitor titre is not due to the presence of anti-factor VIII antibodies undetectable by the Bethesda assay. Haemophilia. 2001; 7:13–19. PMID: 11136375.
Article18. Yoshioka A, Shima M, Fukutake K, Takamatsu J, Shirahata A. Safety and efficacy of a new recombinant FVIII formulated with sucrose (rFVIII-FS) in patients with haemophilia A: a long-term, multicentre clinical study in Japan. Haemophilia. 2001; 7:242–249. PMID: 11380627.
Article19. Windyga J, Rusen L, Gruppo R, et al. BDDrFVIII (Moroctocog alfa [AF-CC]) for surgical haemostasis in patients with haemophilia A: results of a pivotal study. Haemophilia. 2010; 16:731–739. PMID: 20412322.
Article20. Takedani H. Continuous infusion during total joint arthroplasty in Japanese haemophilia A patients: comparison study among two recombinants and one plasma-derived factor VIII. Haemophilia. 2010; 16:740–746. PMID: 20398072.
Article21. Belgaumi AF, Patrick CC, Deitcher SR. Stability and sterility of a recombinant factor VIII concentrate prepared for continuous infusion administration. Am J Hematol. 1999; 62:13–18. PMID: 10467271.
Article22. Parti R, Ardosa J, Yang L, Mankarious S. In vitro stability of recombinant human factor VIII (Recombinate). Haemophilia. 2000; 6:513–522. PMID: 11012695.
Article23. Parti R, Schoppmann A, Lee H, Yang L. Stability of lyophilized and reconstituted plasma/albumin-free recombinant human factor VIII (ADVATE rAHF-PFM). Haemophilia. 2005; 11:492–496. PMID: 16128893.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Autoplex Treatment in a Hemophilia A Patient with High Titer of Anticoagulant FVIII Antibody
- Anesthetic Management of Open Heart Surgery in a Patient with Hemophilia A: A case report
- Factor VIII inhibitors in Korean hemophiliacs-I. prevalence of factor VIII inhibitors
- Supracondylar Fracture of the Humerus in a Hemophiliac with Antibodies to Factor VIII - A Case Report
- A case of prenatal diagnosis of hemophilia A