Ann Dermatol.
2014 Aug;26(4):469-473. 10.5021/ad.2014.26.4.469.
Possible Existence of Melanocytes or Melanoblasts in Human Sebaceous Glands
- Affiliations
-
- 1Department of Dermatology, Kyungpook National University School of Medicine, Daegu, Korea. weonju@knu.ac.kr
- 2Department of Pathology, Keimyung University School of Medicine, Daegu, Korea.
- KMID: 2265587
- DOI: http://doi.org/10.5021/ad.2014.26.4.469
Abstract
- BACKGROUND
Melanocytes are present in both basal epidermis and hair follicles. Melanocyte stem cells have been found in hair follicle bulge. During embryogenesis, the outer cells of the bulge differentiate into the sebaceous gland (SG) and proliferate.
OBJECTIVE
To identify and determine the distribution and morphological characteristics of melanocytes in human SGs.
METHODS
A total of 171 biopsy specimens of face and scalp were studied. Of these specimens, 103 samples contained SGs. We conducted a retrospective review of slides stained with H&E, F-M, anti-S100, anti-c-kit, anti-HMB-45, anti-CD1a, anti-MITF, and anti-tyrosinase. The presence and distribution of melanocytes in human SGs was also evaluated by electron microscopy. In addition, melanocytes were isolated from SGs for primary culture.
RESULTS
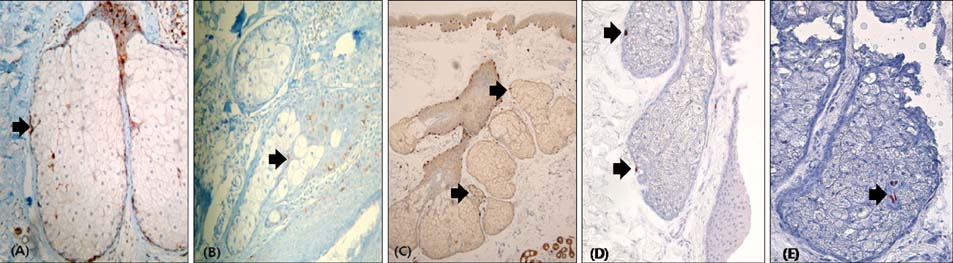
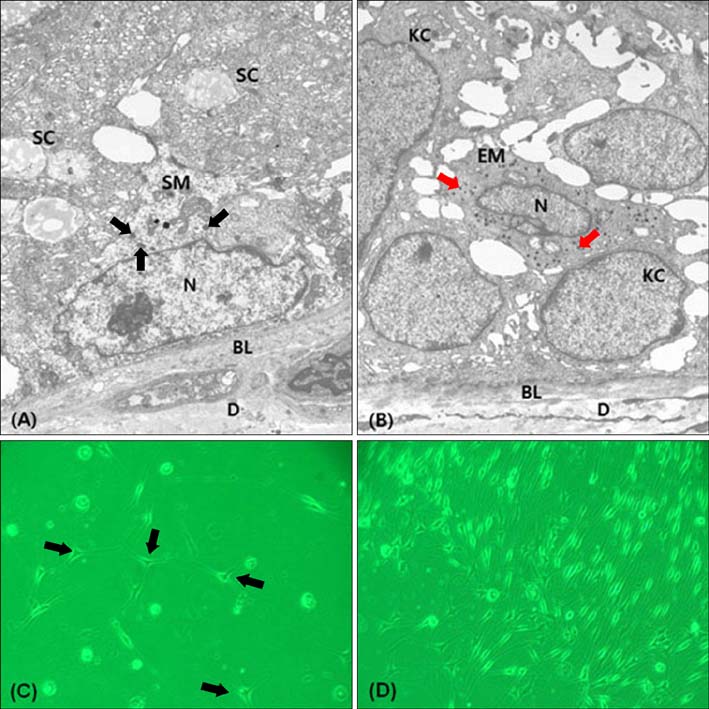
S-100-positive cells were observed mainly at the periphery of SGs in 34 of 54 specimens. We did not find F-M-positive and HMB-45-positive cells in SGs. CD1a-positve cells were identified in two specimens. We also found c-kit-, MITF-, and tyrosinase-positive cells in SGs. Electron micrograph showed the presence of melanocytes in the suprabasal portion of SGs. These melanocytes showed fewer melanin-containing granules than the melanocytes of basal epidermis. However, the individually distributed melanosomes in suprabasal melanocytes were larger than those in epidermal melanocytes. Primary culture of melanocytes derived from SGs showed morphologically homogeneous, slender cell bodies with few dendrites.
CONCLUSION
Our study confirms the presence of non-melanogenic melanocytes and Langerhans cells in human SGs. In addition, the characteristics of the melanocytes in SGs were found to be different from those of the epidermal melanocytes.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Skin Pigmentation and Pigmentary Disorders: Focus on Epidermal/Dermal Cross-Talk
Emanuela Bastonini, Daniela Kovacs, Mauro Picardo
Ann Dermatol. 2016;28(3):279-289. doi: 10.5021/ad.2016.28.3.279.
Reference
-
1. Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992; 115:1111–1119.
Article2. Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005; 307:720–724.
Article3. Amoh Y, Li L, Katsuoka K, Hoffman RM. Embryonic development of hair follicle pluripotent stem (hfPS) cells. Med Mol Morphol. 2010; 43:123–127.
Article4. Ohyama M. Hair follicle bulge: a fascinating reservoir of epithelial stem cells. J Dermatol Sci. 2007; 46:81–89.
Article5. Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004; 118:635–648.
Article6. Tímár J, Udvarhelyi N, Bánfalvi T, Gilde K, Orosz Z. Accuracy of the determination of S100B protein expression in malignant melanoma using polyclonal or monoclonal antibodies. Histopathology. 2004; 44:180–184.
Article7. Ito K, Sato S, Nishijima A, Hiraga K, Hidano A. Melanogenic melanocytes in human sebaceous glands. Experientia. 1976; 32:511–512.
Article8. Bellei B, Pitisci A, Catricalà C, Larue L, Picardo M. Wnt/β-catenin signaling is stimulated by α-melanocyte-stimulating hormone in melanoma and melanocyte cells: implication in cell differentiation. Pigment Cell Melanoma Res. 2011; 24:309–325.
Article9. Luger TA, Brzoska T, Böhm M, Schiller M, Fisbeck T, Scholzen T. The effect of ultraviolet light onthe production and processing of proopiomelanocortin in the skin. In : Ortonne JP, Ballotti R, editors. Mechanisms of suntanning. London: Martin Dunitz;2002. p. 135–142.10. Ito S. Melanins seem to be everywhere in the body, but for what? Pigment Cell Melanoma Res. 2009; 22:12–13.
Article11. Randhawa M, Huff T, Valencia JC, Younossi Z, Chandhoke V, Hearing VJ, et al. Evidence for the ectopic synthesis of melanin in human adipose tissue. FASEB J. 2009; 23:835–843.
Article12. Hirobe T. How are proliferation and differentiation of melanocytes regulated? Pigment Cell Melanoma Res. 2011; 24:462–478.
Article13. Tsatmali M, Ancans J, Thody AJ. Melanocyte function and its control by melanocortin peptides. J Histochem Cytochem. 2002; 50:125–133.
Article14. Whang SW, Lee SE, Kim JM, Kim HJ, Jeong SK, Zouboulis CC, et al. Effects of α-melanocyte-stimulating hormone on calcium concentration in SZ95 sebocytes. Exp Dermatol. 2011; 20:19–23.
Article15. Böhm M, Schiller M, Ständer S, Seltmann H, Li Z, Brzoska T, et al. Evidence for expression of melanocortin-1 receptor in human sebocytes in vitro and in situ. J Invest Dermatol. 2002; 118:533–539.
Article