Ann Dermatol.
2010 May;22(2):138-142. 10.5021/ad.2010.22.2.138.
A Clinical Trial of Combination Therapy with Etanercept and Low Dose Cyclosporine for the Treatment of Refractory Psoriasis
- Affiliations
-
- 1Department of Dermatology, School of Medicine, Kyung Hee University, Seoul, Korea. nikim@khmc.or.kr
- KMID: 2265382
- DOI: http://doi.org/10.5021/ad.2010.22.2.138
Abstract
- BACKGROUND
Over the past decade, combination therapies have become a mainstay of dermatologic care in psoriasis. Combination therapies are often more effective and safer than large dose single-agent therapies. With the emergence of new biologic therapies, dermatologists now have a wider array of tools to treat psoriasis. Although much data exists regarding cyclosporine or biologic agents alone for psoriasis, little is known about the efficacy, safety and tolerability of combination regimens.
OBJECTIVE
We designed a study to evaluate the efficacy and safety of etanercept and cyclosporin combination therapy in patients with refractory psoriasis.
METHODS
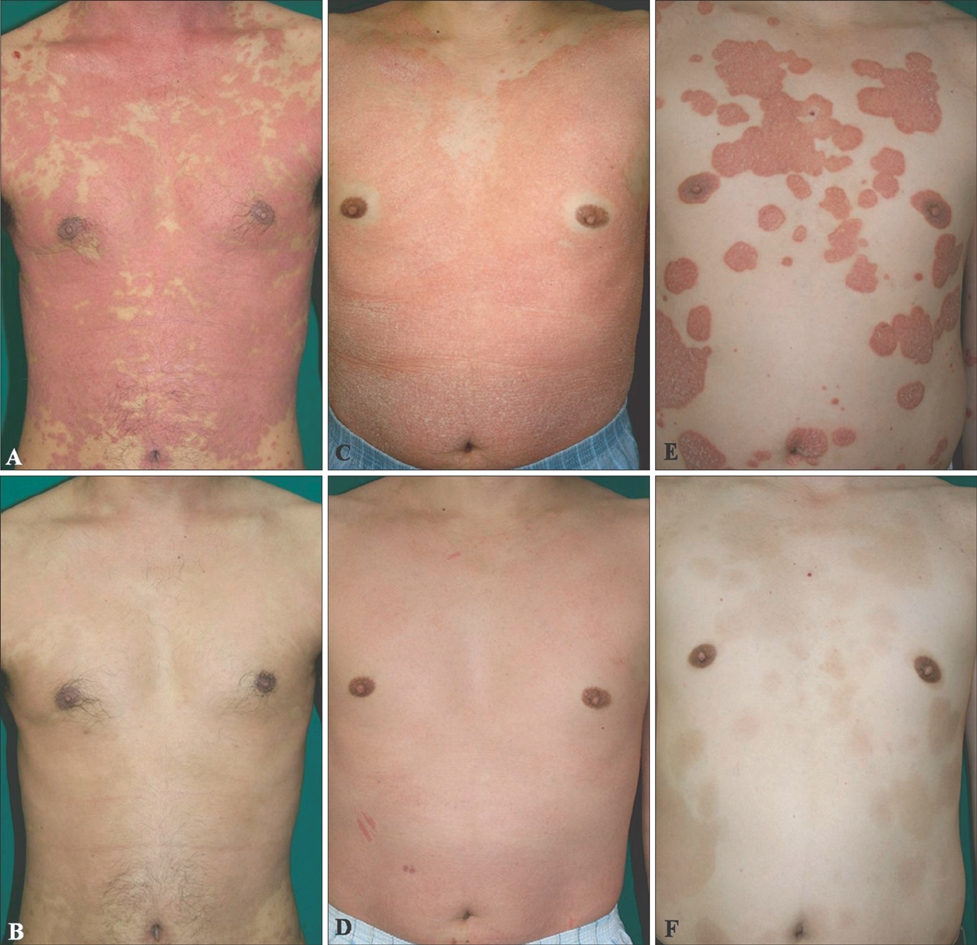
We administered oral cyclosporine (200 mg daily) and subcutaneous etanercept 50 mg weekly injections until symptoms improved, then maintained treatment at a reduced dose. Seven patients with refractory psoriasis were evaluated 4 weekly. RESULTS: All 7 patients showed rapid responses to combination therapy. Mean Psoriasis Area and Severity Index reductions following conditioning therapy (mean: 6.85 weeks) and maintenance therapy (mean: 56.5 weeks) were 94.9% and 93.2%, respectively.
CONCLUSION
Etanercept and low-dose cyclosporine combination therapy appears to be a safe and efficacious alternative treatment strategy for patients with refractory psoriasis. The combination induced rapid improvement in patients with refractory psoriasis and dramatically improved their quality of life. Clinical studies including larger patient cohort are required to validate the safety and efficacy of this combination therapy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Erythrodermic Psoriasis Treated with Golimumab: A Case Report
Won-Ku Lee, Gun-Wook Kim, Hyun-Ho Cho, Won-Jeong Kim, Je-Ho Mun, Margaret Song, Hoon-Soo Kim, Hyun-Chang Ko, Moon-Bum Kim, Byung-Soo Kim
Ann Dermatol. 2015;27(4):446-449. doi: 10.5021/ad.2015.27.4.446.
Reference
-
1. Gudijonsson JE, Elder JT. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Psoriasis. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;169–198.2. Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003. 139:1627–1632.
Article3. Stebbins WG, Lebwohl MG. Biologics in combination with nonbiologics: efficacy and safety. Dermatol Ther. 2004. 17:432–440.
Article4. Lago E, Carneiro S, Cuzzi T, Magalhaes G, Cassia F, Pessanha F, et al. Clinical and immunohistochemical assessment of the effect of cyclosporin in keratinocytes and dermal dendrocytes in psoriasis. J Cutan Pathol. 2007. 34:15–21.
Article5. Berth-Jones J. The use of ciclosporin in psoriasis. J Dermatolog Treat. 2005. 16:258–277.
Article6. Goldsmith DR, Wagstaff AJ. Etanercept: a review of its use in the management of plaque psoriasis and psoriatic arthritis. Am J Clin Dermatol. 2005. 6:121–136.7. Isnard Bagnis C, Tezenas du Montcel S, Beaufils H, Jouanneau C, Jaudon MC, Maksud P, et al. Long-term renal effects of low-dose cyclosporine in uveitis-treated patients: follow-up study. J Am Soc Nephrol. 2002. 13:2962–2968.
Article8. Lowe NJ, Wieder JM, Rosenbach A, Johnson K, Kunkel R, Bainbridge C, et al. Long-term low-dose cyclosporine therapy for severe psoriasis: effects on renal function and structure. J Am Acad Dermatol. 1996. 35:710–719.
Article9. Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003. 49:S118–S124.
Article10. Costanzo A, Talamonti M, Spallone G, Botti E, Chimenti MS, Papoutsaki M, et al. Efficacy of short-term cyclosporine treatment to control psoriasis-related events during efalizumab therapy. Dermatology. 2009. 218:146–150.
Article11. Bellisai F, Giannitti C, Donvito A, Galeazzi M. Combination therapy with cyclosporine A and anti-TNF-alpha agents in the treatment of rheumatoid arthritis and concomitant hepatitis C virus infection. Clin Rheumatol. 2007. 26:1127–1129.
Article12. Iyer S, Yamauchi P, Lowe NJ. Etanercept for severe psoriasis and psoriatic arthritis: observations on combination therapy. Br J Dermatol. 2002. 146:118–121.
Article13. Gul U, Gonul M, Kilic A, Erdem R, Cakmak SK, Gunduz H. Treatment of psoriatic arthritis with etanercept, methotrexate, and cyclosporin A. Clin Ther. 2006. 28:251–254.
Article14. Javier P, Esteban D, Tatiana S, Amaro G. Cyclosporin A effectively controls recalcitrant psoriasis resistant to biologic therapy. J Am Acad Dermatol. 2007. 56:AB180.15. Smith KJ, Skelton HG. Rapid onset of cutaneous squamous cell carcinoma in patients with rheumatoid arthritis after starting tumor necrosis factor alpha receptor IgG1-Fc fusion complex therapy. J Am Acad Dermatol. 2001. 45:953–956.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Refractory Plaque Psoriasis Treated with a Combination of Etanercept and Low Dose Methotrexate
- Real-World Experience with Etanercept Therapy and the Switching Pattern among Korean Patients with Psoriasis after Withdrawal of Etanercept
- Low-dose Acitretin and Cyclosporine Combination Therapy in Patients with Psoriasis: A Retrospective Analysis of 37 Cases
- Comparative Study of Quality of Life in Patients with Scalp Psoriasis Treated Using Topical Steroid Alone vs. Topical Steroid Combined with Cyclosporine
- Three Cases of Refractory Psoriasis Treated with Low-dose Azathioprine and Acitretin Combined Therapy