Ann Dermatol.
2010 Aug;22(3):284-289. 10.5021/ad.2010.22.3.284.
The Immunohistochemical Patterns of the beta-Catenin Expression in Pilomatricoma
- Affiliations
-
- 1Department of Dermatology, College of Medicine, Yeungnam University, Daegu, Korea. khkim@med.yu.ac.kr
- KMID: 2265361
- DOI: http://doi.org/10.5021/ad.2010.22.3.284
Abstract
- BACKGROUND
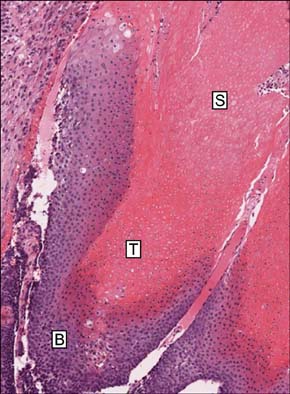
Pilomatricoma is a benign follicular tumor that is composed of basaloid cells, transitional cells and shadow cells. beta-Catenin is a 92-kDa protein, and it plays important roles in cell-cell adhesion at the cell membrane and signal transduction in the nucleus. beta-Catenin has recently been shown to play an important role in the formation of hair follicle-related tumors, including pilomatricoma. However, the pattern and the intracellular localization of the beta-Catenin expression are still controversial.
OBJECTIVE
We wanted to evaluate the pattern and the intracellular localization of the beta-Catenin expression in pilomatricoma by performing immunohistochemical staining.
METHODS
Twenty-seven paraffin-embedded tissue samples that were diagnosed as pilomatricoma were immunohistochemically stained with beta-Catenin antibody.
RESULTS
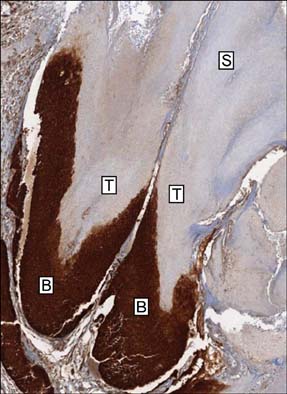
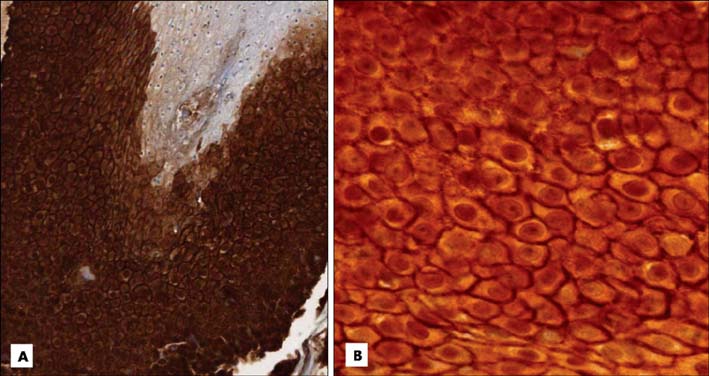
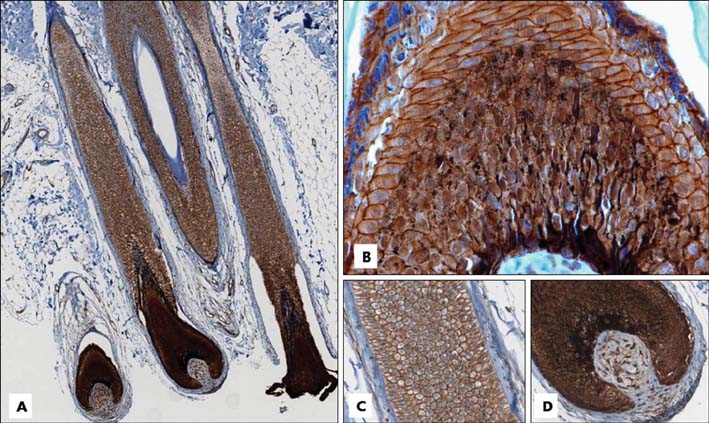
Basaloid cells were found 15 samples of the total 27 pilomatricomas. All (15/15) of the basaloid cells strongly expressed beta-Catenin, but the transitional cells and the shadow cells did not. In the basaloid cells, the nuclei and membranes showed prominent beta-Catenin immunoreactivities, but the cytoplasm showed weak beta-Catenin immunoreactivity.
CONCLUSION
This study confirmed that the nucleus and membrane of all the basaloid cells in the pilomatricomas showed a strong beta-Catenin expression, but the transitional cells and shadow cells showed negative beta-Catenin immunoreactivity.
MeSH Terms
Figure
Reference
-
1. Taylor RS, Perone JB, Kaddu S, Kerl H. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Appendage tumors and hamartomas of the skin. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1081–1082.2. Choi YD, Park JN, Kang MS, Cho SH, Park SW. Expressions of cytokeratin and Ki-67 in the development of the pilomatricoma. Korean J Dermatol. 2003. 41:1619–1626.3. Huber AH, Weis WI. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell. 2001. 105:391–402.
Article4. Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000. 148:399–404.
Article5. Barker N, Clevers H. Catenins, Wnt signaling and cancer. Bioessays. 2000. 22:961–965.
Article6. Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998. 95:605–614.
Article7. Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999. 21:410–413.
Article8. Durand M, Moles JP. Beta-catenin mutations in a common skin cancer: pilomatricoma. Bull Cancer. 1999. 86:725–726.9. Moreno-Bueno G, Gamallo C, Perez-Gallego L, Contreras F, Palacios J. β-catenin expression in pilomatrixomas. Relationship with β-catenin gene mutations and comparison with β-catenin expression in normal hair follicles. Br J Dermatol. 2001. 145:576–581.
Article10. Park SW, Suh KS, Wang HY, Kim ST, Sung HS. β-Catenin expression in the transitional cell zone of pilomatricoma. Br J Dermatol. 2001. 145:624–629.
Article11. Hassanein AM, Glanz SM, Kessler HP, Eskin TA, Liu C. β-Catenin is expressed aberrantly in tumors expressing shadow cells. Pilomatricoma, craniopharyngioma, and calcifying odontogenic cyst. Am J Clin Pathol. 2003. 120:732–736.
Article12. Xia J, Urabe K, Moroi Y, Koga T, Duan H, Li Y, Furue Y. beta-Catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci. 2006. 41:67–75.
Article13. Demirkan NC, Bir F, Erdem O, Düzcan E. Immunohistochemical expression of β-catenin, E-cadherin, cyclin D1 and c-myc in benign trichogenic tumors. J Cutan Pathol. 2007. 34:467–473.
Article14. Hashimoto K, Nelson RG, Lever WF. Calcifying epithelioma of Malherbe. Histochemical and electron microscopic studies. J Invest Dermatol. 1966. 46:391–408.15. Seo SH, Jeong JT, Kye YC, Kim SN. The study of the clinical, histopathological and pathogenetic feature of pilomatricoma. Korean J Dermatol. 2001. 39:1275–1285.16. Ackerman AB, Boer A, Bennin B, Gottlieb GJ. Histologic diagnosis of inflammatory skin disease: an algorithmic method based on pattern analysis. 2005. 3rd ed. New York: Ardor Scribendi;147.17. Lee CW, Jang HS, Oh CK, Kwon KS. Apoptosis and expression of bcl-2, p53, and Ki-67 in pilomatricoma. Korean J Dermatol. 1999. 37:1560–1566.18. Min KS, Shin JH, Lee HG, Kim JM. The immunohistochemical study of bcl-2 and p53 expression of pilomatricoma. Korean J Dermatol. 2000. 38:38–44.19. Forbis R Jr, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961. 83:606–618.
Article20. Jang HZ, Baik YG, Kim JM, Sohn JH. The study of the clinical and histopatholgical features of pilomatricoma. Korean J Dermatol. 1997. 35:693–701.21. Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999. 341:491–497.
Article22. Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004. 303:1483–1487.
Article23. Hassanein AM, Glanz SM. β-catenin expression in benign and malignant pilomatrix neoplasms. Br J Dermatol. 2004. 150:511–516.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of E-cadherin/Beta-catenin Complex and Cyclin D1 in Head and Neck Squamous Cell Carcinoma
- Expression of beta-catenin and Adenomatous Polyposis Coli(APC) Protein in Squamous Cell Carcinoma of the Laryngeal Cancers
- Abnormalities of beta-Catenin Gene in Pilomatricomas
- Immunohistochemical Study of beta-catenin Expression between Hepatocellular Carcinoma and Cholangiocarcinoma
- RImmunohistochemical Evaluation of E-cadherin/catenin (alpha-, beta-, gamma-catenin and p120CTN) Complex Expression in Early Gastric Cancer