Anat Cell Biol.
2013 Jun;46(2):149-156. 10.5115/acb.2013.46.2.149.
Influence of developing ligaments on the muscles in contact with them: a study of the annular ligament of the radius and the sacrospinous ligament in mid-term human fetuses
- Affiliations
-
- 1Medical Education Center, Aichi Medical University School of Medicine, Aichi, Japan. sho5-884@umin.ac.jp
- 2Department of Anatomy, Aichi Medical University School of Medicine, Aichi, Japan.
- 3Department of Anatomy, Chonbuk National University Medical School, Jeonju, Korea.
- 4Department of Anatomy and Embryology II, Complutense University School of Medicine, Madrid, Spain.
- 5Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Hokkaido, Japan.
- KMID: 2263107
- DOI: http://doi.org/10.5115/acb.2013.46.2.149
Abstract
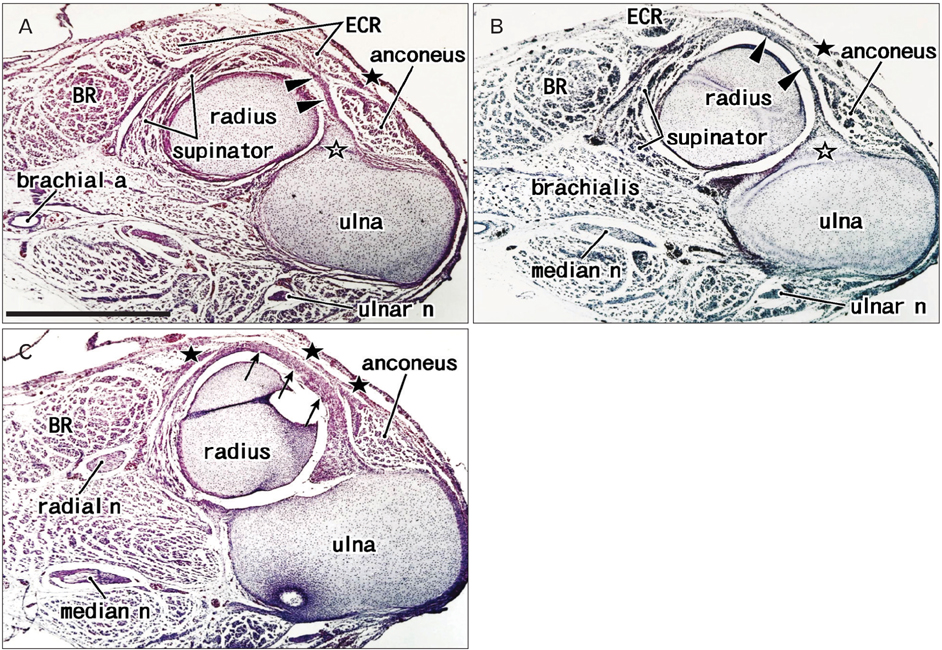
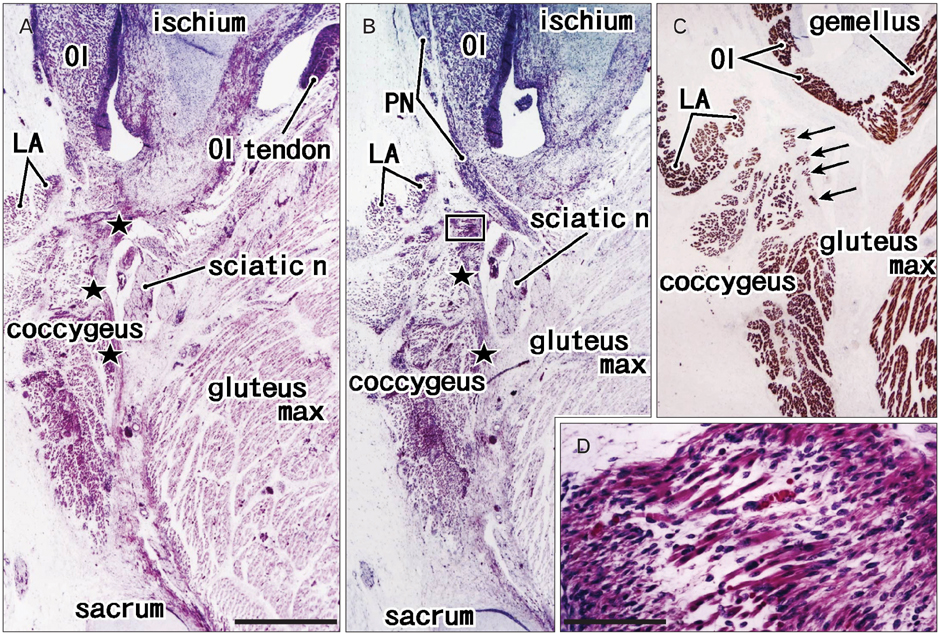
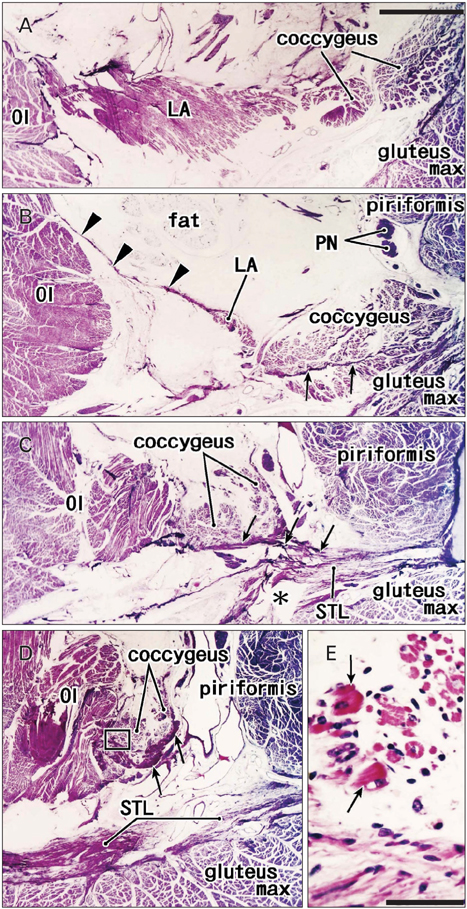
- The supinator muscle originates from the annular ligament of the radius, and the muscle fibers and ligament take a similar winding course. Likewise, the coccygeus muscle and the sacrospinous ligament are attached together, and show a similar fiber orientation. During dissection of adult cadavers for our educational curriculum, we had the impression that these ligaments grow in combination with degeneration of parts of the muscles. In histological sections of 25 human fetuses at 10-32 weeks of gestation, we found that the proximal parts of the supinator muscle were embedded in collagenous tissue when the developing annular ligament of the radius joined the thick intermuscular connecting band extending between the extensor carpi radialis and anconeus muscles at 18-22 weeks of gestation, and the anterior parts of the coccygeus muscle were surrounded by collagenous tissue when the intramuscular tendon became the sacrospinous ligament at 28-32 weeks. Parts of these two muscles each seemed to provide a mold for the ligament, and finally became involved with it. This may be the first report to indicate that a growing ligament has potential to injure parts of the "mother muscle," and that this process may be involved in the initial development of the ligament.
Keyword
MeSH Terms
Figure
Reference
-
1. Fick R. von Bardeleben K, editor. Anatomie der Gelenke. Kreuz-Darmbein-gelenk., Handbuch der Anatomie und Mechanik der Gelenke unter Berűcksichtigung der bewegeden Muskeln. 1904. Vol. 2. Jena: Gustav Fischer;289–303.2. Harrison DE, Harrison DD, Troyanovich SJ. The sacroiliac joint: a review of anatomy and biomechanics with clinical implications. J Manipulative Physiol Ther. 1997. 20:607–617.3. Hammer N, Steinke H, Slowik V, Josten C, Stadler J, Böhme J, Spanel-Borowski K. The sacrotuberous and the sacrospinous ligament: a virtual reconstruction. Ann Anat. 2009. 191:417–425.4. Henle J. Merkel F, editor. Bānder der unteren Extemitāt. Grundriss der Anatomie des Menschen. 1900. 3rd ed. Braunschweig: Friedrich Vieweg und Sohn;71.5. Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, Magnusson SP. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009. 19:500–510.6. Thomopoulos S, Genin GM, Galatz LM. The development and morphogenesis of the tendon-to-bone insertion: what development can teach us about healing. J Musculoskelet Neuronal Interact. 2010. 10:35–45.7. Mackey AL, Heinemeier KM, Koskinen SO, Kjaer M. Dynamic adaptation of tendon and muscle connective tissue to mechanical loading. Connect Tissue Res. 2008. 49:165–168.8. Nowlan NC, Bourdon C, Dumas G, Tajbakhsh S, Prendergast PJ, Murphy P. Developing bones are differentially affected by compromised skeletal muscle formation. Bone. 2010. 46:1275–1285.9. Rot-Nikcevic I, Reddy T, Downing KJ, Belliveau AC, Hallgrímsson B, Hall BK, Kablar B. Myf5-/- :MyoD-/- amyogenic fetuses reveal the importance of early contraction and static loading by striated muscle in mouse skeletogenesis. Dev Genes Evol. 2006. 216:1–9.10. Mahasen LM, Sadek SA. Developmental morphological and histological studies on structures of the human fetal elbow joint. Cells Tissues Organs. 2000. 166:359–372.11. Gray DJ, Gardner E. Prenatal development of the human elbow joint. Am J Anat. 1951. 88:429–469.12. Mérida-Velasco JA, Sánchez-Montesinos I, Espín-Ferra J, Mérida-Velasco JR, Rodríguez-Vázquez JF, Jiménez-Collado J. Development of the human elbow joint. Anat Rec. 2000. 258:166–175.13. Reidenbach MM, Schmidt HM. Prenatal development of the radial annular ligament. Ann Anat. 1993. 175:459–467.14. Andersen H. Histochemical studies of the histogenesis of the human elbow joint. Acta Anat (Basel). 1962. 51:50–68.15. Bardeen CR. Development and variation of the nerves and the musculature of the inferior extremity and of the neighboring regions of the trunk in man. Am J Anat. 1906. 6:259–390.16. Niikura H, Jin ZW, Cho BH, Murakami G, Yaegashi N, Lee JK, Lee NH, Li CA. Human fetal anatomy of the coccygeal attachments of the levator ani muscle. Clin Anat. 2010. 23:566–574.17. Power RM. Embryological development of the levator ani muscle. Am J Obstet Gynecol. 1948. 55:367–381.18. Abe H, Ishizawa A, Cho KH, Suzuki R, Fujimiya M, Rodríguez-Vázquez JF, Murakami G. Fetal development of the transverse atlantis and alar ligaments at the craniovertebral junction. Clin Anat. 2012. 25:714–721.19. Kim JH, Abe S, Shibata S, Asakawa S, Maki H, Murakami G, Cho BH. Dense distribution of macrophages in flexor aspects of the hand and foot of mid-term human fetuses. Anat Cell Biol. 2012. 45:259–267.20. O'Driscoll SW, Horii E, Morrey BF, Carmichael SW. Anatomy of the ulnar part of the lateral collateral ligament of the elbow. Clin Anat. 1992. 5:296–303.21. Hannouche D, Bégué T. Functional anatomy of the lateral collateral ligament complex of the elbow. Surg Radiol Anat. 1999. 21:187–191.22. Imatani J, Ogura T, Morito Y, Hashizume H, Inoue H. Anatomic and histologic studies of lateral collateral ligament complex of the elbow joint. J Shoulder Elbow Surg. 1999. 8:625–627.23. O'Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991. 73:440–446.24. Kim PT, Isogai S, Murakami G, Wada T, Aoki M, Yamashita T, Ishii S. The lateral collateral ligament complex and related muscles act as a dynamic stabilizer as well as a static supporting structure at the elbow joint: an anatomical and experimental study. Okajimas Folia Anat Jpn. 2002. 79:55–61.25. Hast MH, Perkins RE. Secondary tensor and supinator muscles of the human proximal radio-ulnar joint. J Anat. 1986. 146:45–51.26. Dujardin FH, Roussignol X, Hossenbaccus M, Thomine JM. Experimental study of the sacroiliac joint micromotion in pelvic disruption. J Orthop Trauma. 2002. 16:99–103.27. Seizeur R, Forlodou P, Person H, Morin JF, Sénécail B. The morphometric study of the sacrospinal and sacrotuberal ligaments correlated with the morphometry of the pelvis. Surg Radiol Anat. 2005. 27:517–523.28. Simonian PT, Routt ML Jr, Harrington RM, Tencer AF. Anterior versus posterior provisional fixation in the unstable pelvis. A biomechanical comparison. Clin Orthop Relat Res. 1995. (310):245–251.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Topographical anatomy of the palmar carpal ligaments in Korean adults
- Clinical study of the sacrospinous ligament suspension using Miya hook in management of pelvic organ prolapse
- Ultrasonographic diagnosis of snapping annular ligament in the elbow
- MRI of Spontaneous Reduction of an Entrapped Annular Ligament in an Atypical Pulled Elbow Patient: A Case Report
- Mechanical Properties of Palmar Radiocarpal Ligaments of Wrist and Their Clinical significances