Allergy Asthma Immunol Res.
2013 Nov;5(6):397-401. 10.4168/aair.2013.5.6.397.
The Effects of Storage Conditions on the Stability of House Dust Mite Extracts
- Affiliations
-
- 1Departmet of Internal Medicine and Institute of Allergy, Yonsei University College of Medicine, Seoul, Korea. parkjw@yuhs.ac
- 2Center for Immunology and Pathology, Korea National Institute of Health, Seoul, Korea.
- KMID: 2260284
- DOI: http://doi.org/10.4168/aair.2013.5.6.397
Abstract
- PURPOSE
Allergen extracts from the house dust mite (HDM, Dermatophagoides pteronyssinus) are widely utilized for diagnosis and treatment of allergic diseases. It is known that allergen extracts degrade and lose potency when stored over time.
METHODS
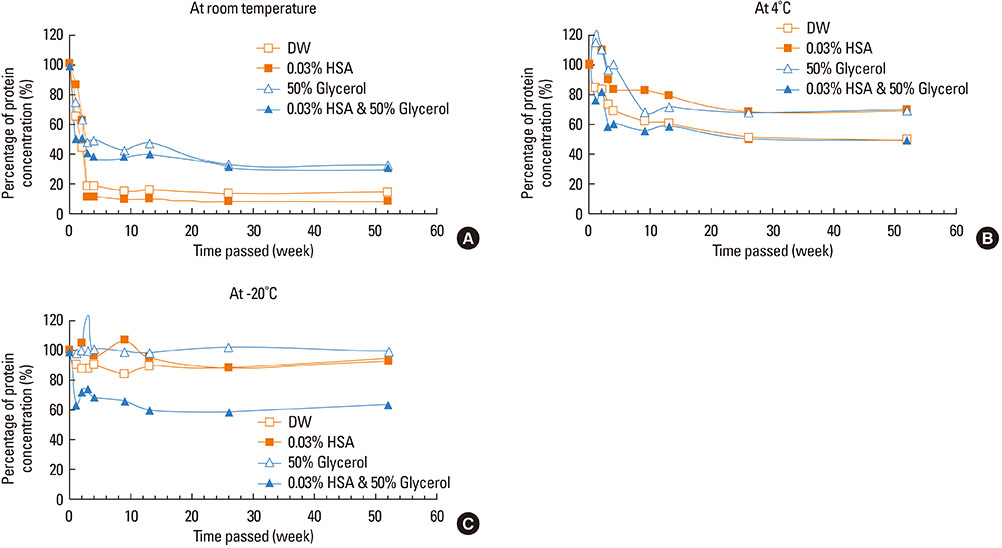
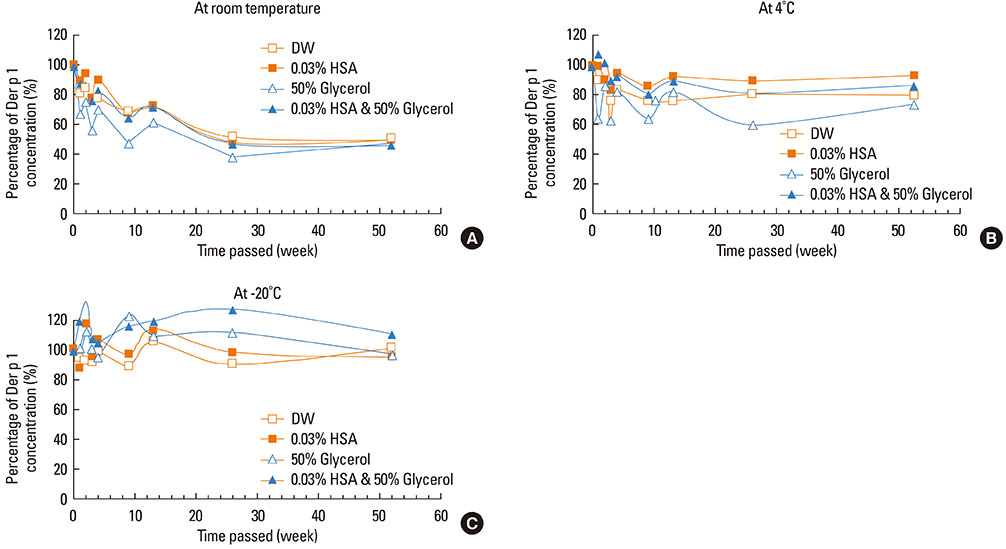
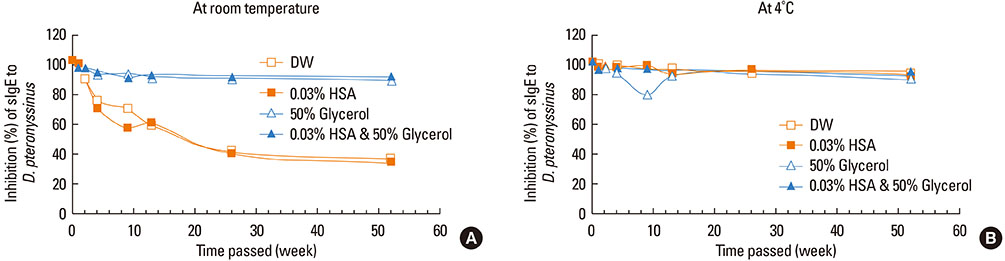
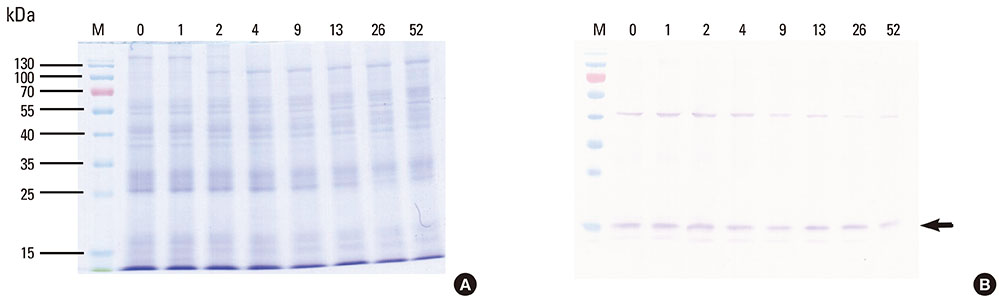
This study aimed to determine the optimal conditions for stability of allergen extracts. This study was undertaken to investigate the optimal storage conditions for HDM extracts, the effects of adding 0.03% human serum albumin (HSA) and 50% glycerol were evaluated at -20degrees C, 4degrees C, and room temperature (RT). Changes in protein and group 1 major allergen (Der p 1) concentration, as well as allergenicity were measured over a 1 year period using the Bradford assay, two-site ELISA, and ELISA inhibition.
RESULTS
Protein concentrations decreased by 86%, 51%, and 6% at RT, 4degrees C, and -20degrees C, respectively, when stored in distilled water. Overall allergenicity remained high (89.9%) when the extracts was reconstituted in 50% glycerol solution, and was 93.1% when reconstituted in 50% glycerol and 0.03% HSA at RT. Allergenicity was decreased to 36.6% and 33.3%, however, reconstitution in DW or 0.03% HSA solution at RT, respectively. Allergenicity was remained high as 92.0%-97.0% when stored at 4degrees C regardless of the buffer conditions.
CONCLUSIONS
Storage temperature is the most important factor in preserving allergenicity of HDM extracts, which is ideal at 4degrees C. The addition of 50% glycerol to the storage buffer was also found to play an important role in increasing the shelf-life of HDM extracts at RT.
Keyword
MeSH Terms
Figure
Reference
-
1. Jeong KY, Park JW, Hong CS. House dust mite allergy in Korea: the most important inhalant allergen in current and future. Allergy Asthma Immunol Res. 2012; 4:313–325.2. Thomas WR. House dust allergy and immunotherapy. Hum Vaccin Immunother. 2012; 8:1469–1478.3. Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011; 52:393–400.4. Jeong KY, Kim C, Yong TS. Enzymatic activities of allergen extracts from three species of dust mites and cockroaches commonly found in Korean home. Korean J Parasitol. 2010; 48:151–155.5. Center JG, Shuller N, Zeleznick LD. Stability of antigen E in commercially prepared ragweed pollen extracts. Dev Biol Stand. 1975; 29:114–122.6. Nelson HS. The effect of preservatives and dilution on the deterioration of Russian thistle (Salsola pestifer), a pollen extracts. J Allergy Clin Immunol. 1979; 63:417–425.7. Anderson MC, Baer H. Antigenic and allergenic changes during storage of a pollen extracts. J Allergy Clin Immunol. 1982; 69:3–10.8. Bousquet J, Djoukadar F, Hewitt B, Guerin B, Michel FB. Comparison of the stability of a mite and a pollen extract stored in normal conditions of use. Clin Allergy. 1985; 15:29–35.9. Naerdal A, Vilsvik JS. Stabilization of a diluted aqueous mite allergen preparation by addition of human serum albumin. An intracutaneous test study. Clin Allergy. 1983; 13:149–153.10. Jeong KY, Choi SY, Lee JH, Lee IY, Yong TS, Lee JS, Hong CS, Park JW. Standardization of house dust mite extracts in Korea. Allergy Asthma Immunol Res. 2012; 4:346–350.11. Hoff M, Krail M, Kästner M, Haustein D, Vieths S. Fusarium culmorum causes strong degradation of pollen allergens in extracts mixtures. J Allergy Clin Immunol. 2002; 109:96–101.12. Soldatova LN, Paupore EJ, Burk SH, Pastor RW, Slater JE. The stability of house dust mite allergens in glycerinated extracts. J Allergy Clin Immunol. 2000; 105:482–488.13. Liu T, Lin Y. The epitope stability of group 1 and group 2 allergens in mite extracts. Ann Allergy Asthma Immunol. 1998; 80:177–183.14. Park JW, Kim KS, Jin HS, Kim CW, Kang DB, Choi SY, Yong TS, Oh SH, Hong CS. Der p 2 isoallergens have different allergenicity, and quantification with 2-site ELISA using monoclonal antibodies is influenced by the isoallergens. Clin Exp Allergy. 2002; 32:1042–1047.15. Jeong KY, Lee IY, Yong TS, Lee JH, Kim EJ, Lee JS, Hong CS, Park JW. Sequence polymorphisms of Der f 1, Der p 1, Der f 2 and Der p 2 from Korean house dust mite isolates. Exp Appl Acarol. 2012; 58:35–42.16. Sudha VT, Srivastava D, Arora N, Gaur SN, Singh BP. Stability of protease-rich Periplaneta americana allergen extracts during storage: formulating preservatives to enhance shelf life. J Clin Immunol. 2007; 27:294–301.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repellent effect of Mate tea and Jasmine tea against house dust mites (Dermatophagoides farinae and D. pteronyssinus)
- Distribution of House Dust Mites in the Bedroom of Patients with Allergic Rhinitis in Pusan Area
- Avian Mite Dermatitis: Observation of the Causative Mites and Clinical Findings
- Allergen standardization of whole body extract of Korean house dust mite by in vivo method
- Sensitization of house dust mites in the allergic patients and mite ecology in their house dusts