J Clin Neurol.
2015 Apr;11(2):183-187. 10.3988/jcn.2015.11.2.183.
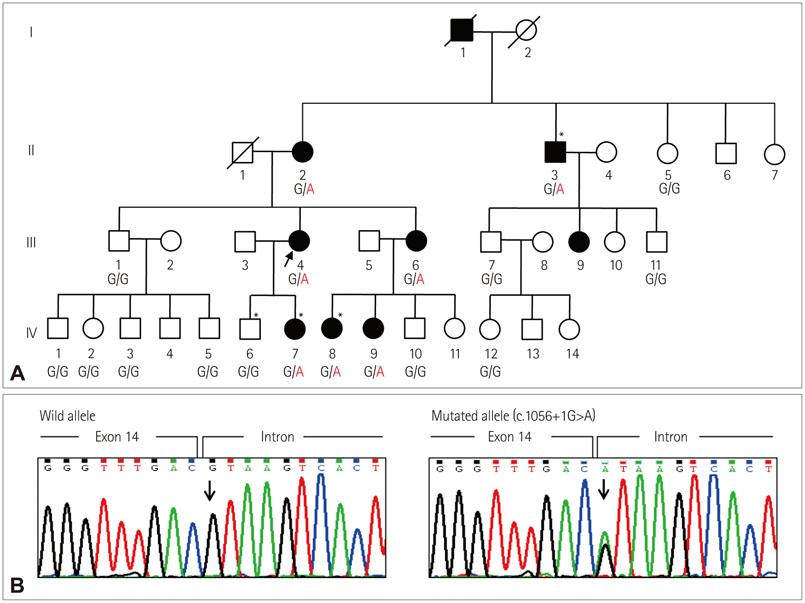
Molecular Genetic Diagnosis of a Bethlem Myopathy Family with an Autosomal-Dominant COL6A1 Mutation, as Evidenced by Exome Sequencing
- Affiliations
-
- 1Department of Neurology, Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea.
- 2Department of Neurology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. bochoi@skku.edu
- 5Department of Biological Science, Kongju National University, Gongju, Korea. kwchung@kongju.ac.kr
- KMID: 2242664
- DOI: http://doi.org/10.3988/jcn.2015.11.2.183
Abstract
- BACKGROUND
We describe herein the application of whole exome sequencing (WES) for the molecular genetic diagnosis of a large Korean family with dominantly inherited myopathy.
CASE REPORT
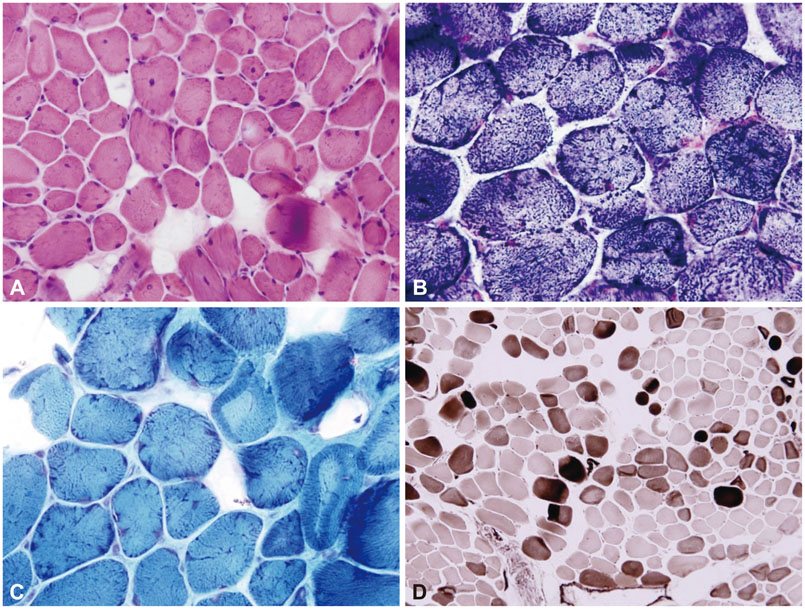
The affected individuals presented with slowly progressive proximal weakness and ankle contracture. They were initially diagnosed with limb-girdle muscular dystrophy (LGMD) based on clinical and pathologic features. However, WES and subsequent capillary sequencing identified a pathogenic splicing-site mutation (c.1056+1G>A) in COL6A1, which was previously reported to be an underlying cause of Bethlem myopathy. After identification of the genetic cause of the disease, careful neurologic examination revealed subtle contracture of the interphalangeal joint in the affected members, which is a characteristic sign of Bethlem myopathy. Therefore, we revised the original diagnosis from LGMD to Bethlem myopathy.
CONCLUSIONS
This is the first report of identification of COL6A1-mediated Bethlem myopathy in Korea, and indicates the utility of WES for the diagnosis of muscular dystrophy.
MeSH Terms
Figure
Reference
-
1. Norwood F, de Visser M, Eymard B, Lochmüller H, Bushby K. EFNS Guideline Task Force. EFNS guideline on diagnosis and management of limb girdle muscular dystrophies. Eur J Neurol. 2007; 14:1305–1312.
Article2. Bushby K. Diagnosis and management of the limb girdle muscular dystrophies. Pract Neurol. 2009; 9:314–323.
Article3. Ku CS, Naidoo N, Pawitan Y. Revisiting Mendelian disorders through exome sequencing. Hum Genet. 2011; 129:351–370.
Article4. Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011; 12:745–755.
Article5. Davidson AE, Siddiqui FM, Lopez MA, Lunt P, Carlson HA, Moore BE, et al. Novel deletion of lysine 7 expands the clinical, histopathological and genetic spectrum of TPM2-related myopathies. Brain. 2013; 136(Pt 2):508–521.
Article6. Klar J, Sobol M, Melberg A, Mäbert K, Ameur A, Johansson AC, et al. Welander distal myopathy caused by an ancient founder mutation in TIA1 associated with perturbed splicing. Hum Mutat. 2013; 34:572–577.7. Ronchi D, Di Fonzo A, Lin W, Bordoni A, Liu C, Fassone E, et al. Mutations in DNA2 link progressive myopathy to mitochondrial DNA instability. Am J Hum Genet. 2013; 92:293–300.
Article8. Weterman MA, Barth PG, van Spaendonck-Zwarts KY, Aronica E, Poll-The BT, Brouwer OF, et al. Recessive MYL2 mutations cause infantile type I muscle fibre disease and cardiomyopathy. Brain. 2013; 136(Pt 1):282–293.
Article9. Choi BO, Koo SK, Park MH, Rhee H, Yang SJ, Choi KG, et al. Exome sequencing is an efficient tool for genetic screening of Charcot-Marie-Tooth disease. Hum Mutat. 2012; 33:1610–1615.
Article10. Pan TC, Zhang RZ, Sudano DG, Marie SK, Bönnemann CG, Chu ML. New molecular mechanism for Ullrich congenital muscular dystrophy: a heterozygous in-frame deletion in the COL6A1 gene causes a severe phenotype. Am J Hum Genet. 2003; 73:355–369.
Article11. Lucioli S, Giusti B, Mercuri E, Vanegas OC, Lucarini L, Pietroni V, et al. Detection of common and private mutations in the COL6A1 gene of patients with Bethlem myopathy. Neurology. 2005; 64:1931–1937.
Article12. Baker NL, Mörgelin M, Pace RA, Peat RA, Adams NE, Gardner RJ, et al. Molecular consequences of dominant Bethlem myopathy collagen VI mutations. Ann Neurol. 2007; 62:390–405.
Article13. Lamandé SR, Shields KA, Kornberg AJ, Shield LK, Bateman JF. Bethlem myopathy and engineered collagen VI triple helical deletions prevent intracellular multimer assembly and protein secretion. J Biol Chem. 1999; 274:21817–21822.
Article14. Lampe AK, Bushby KM. Collagen VI related muscle disorders. J Med Genet. 2005; 42:673–685.
Article15. Scacheri PC, Gillanders EM, Subramony SH, Vedanarayanan V, Crowe CA, Thakore N, et al. Novel mutations in collagen VI genes: expansion of the Bethlem myopathy phenotype. Neurology. 2002; 58:593–602.
Article16. Merlini L, Morandi L, Granata C, Ballestrazzi A. Bethlem myopathy: early-onset benign autosomal dominant myopathy with contractures. Description of two new families. Neuromuscul Disord. 1994; 4:503–511.
Article17. Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010; 11:31–46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Family of Bethlem Myopathy Caused by a Heterozygous COL6A1 Mutation
- A Family of Bethlem Myopathy
- De novo mutations in COL4A5 identified by whole exome sequencing in 2 girls with Alport syndrome in Korea
- Clinical, Pathologic, and Genetic Features of Collagen VI-Related Myopathy in Korea
- Tubulopathy: the clinical and genetic approach in diagnosis