Korean Circ J.
2008 Jun;38(6):305-312. 10.4070/kcj.2008.38.6.305.
Assessment of Left Atrial Function and Remodeling in Patients With Atrial Fibrillation by Performing Strain Echocardiography: A Prospective Study to Assess the Influence of Renin-Angiotensin System Inhibitors on Atrial Fibrillation
- Affiliations
-
- 1Division of Cardiology, Maryknoll Medical Center, Busan, Korea. Kyoungim74@dreamwiz.com
- KMID: 2225777
- DOI: http://doi.org/10.4070/kcj.2008.38.6.305
Abstract
-
BACKGROUND AND OBJECTIVES: Renin-angiotensin system (RAS) inhibitors are likely to reduce the development of atrial fibrillation (AF) by preventing atrial fibrosis. We attempted to assess the relevance of strain echocardiography for quantitative assessment of the left atrial (LA) status in AF, its modification by RAS inhibitors and changes of biochemical markers during cardiac remodeling in AF.
SUBJECTS AND METHODS
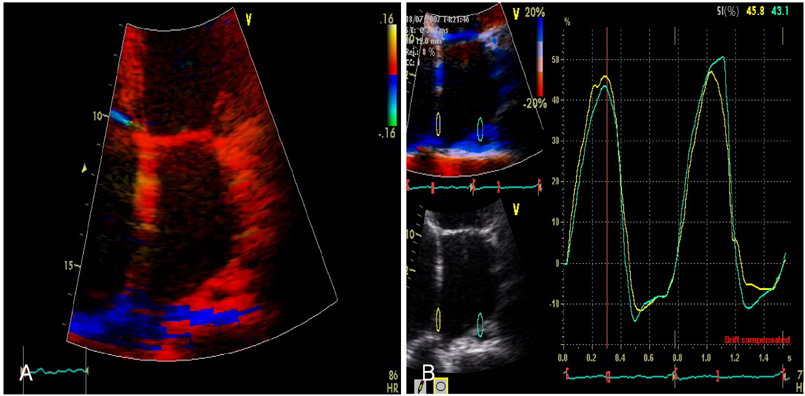
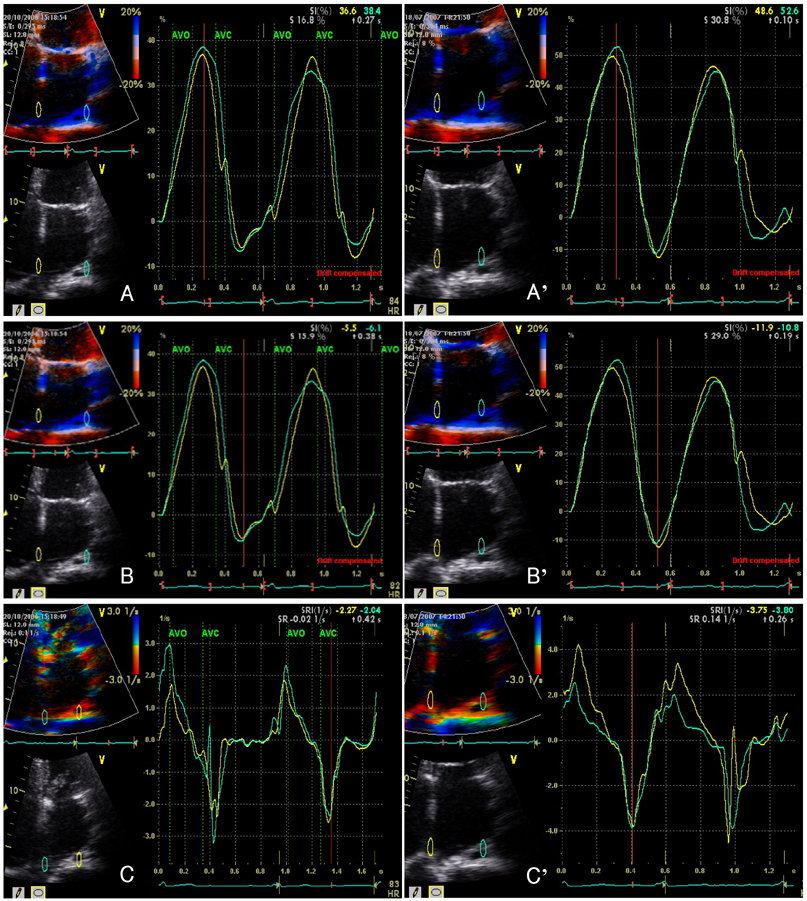
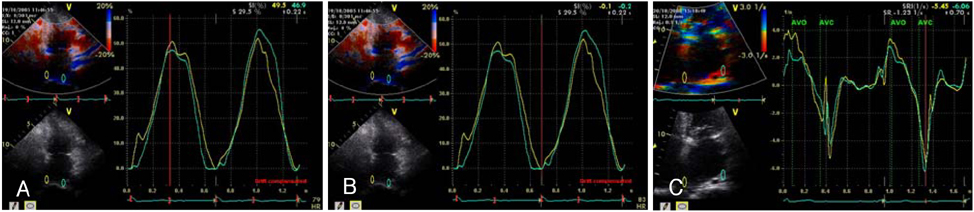
Strain echocardiography is performed 2 times (baseline and 12 month) in 60 patients with AF (RAS inhibitors-used group: 30, non-used group: 30). In an apical 4-chamber view, the regional analysis consisted of placing the region of interest cursor at the basal segments of the septal and lateral wall of LA. Mean peak systolic and early diastolic strain/rate are measured with LA end-systolic antero-posterior, longitudinal and transverse dimensions.
RESULTS
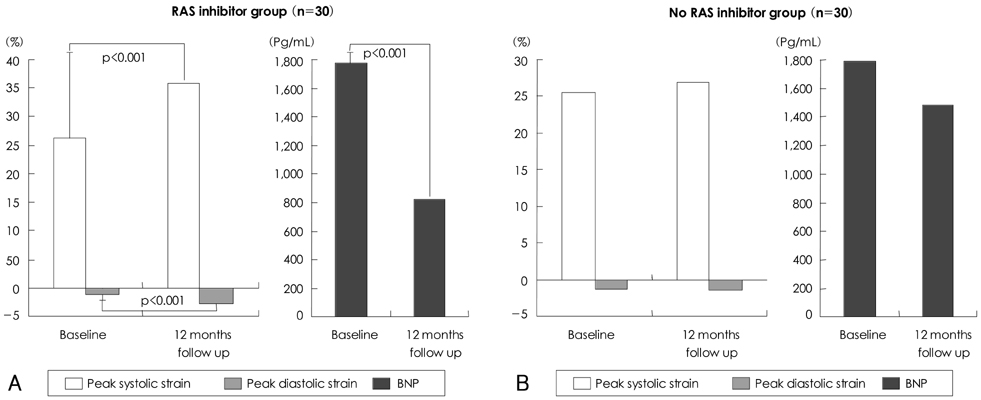
Six patients of RAS inhibitors-used group (group A, 20%) and three patients of non-used group (group B, 10%) were converted to normal sinus rhythm during the study. LA size, E wave velocity, E/E', strain parameters showed no significant differences between groups at the baseline. There were no significant differences in LA size and E wave velocity between groups at the 12 months, however, peak systolic strain/rate were significantly higher in group A (36.71+/-13.63% and 2.98+/-0.59s(-1), p<0.05, respectively) than group B (27.21+/-10.49% and 2.21+/-0.47s(-1)). In addition, peak early diastolic strain/rate were significantly higher in group A (-1.89+/-3.30% and -2.32 +/-0.77s-1 p<0.05, respectively) than group B (-0.83+/-2.79% and -1.77+/-0.25s(-1)). There were no significant differences in C-reactive protein (CRP) and B-type natriuretic peptide (BNP) at the baseline, but BNP were significantly reduced in group A (822.9+/-798.3 pg/mL, p<0.05) than group B (1481.9+/-209.97 pg/mL) at the 12-month follow-up.
CONCLUSION
The increased values of atrial peak systolic and diastolic strain/rate after treatment with RAS inhibitors revealed that passive stretching and shortening of LA wall might improve in some patients with AF even before LA size change possibly because of reduced atrial fibrosis and increased compliance. Our results indicated that strain echocardiography provides clinically useful information of LA function and remodeling and treatment with RAS inhibitors appears to preserve LA reservoir function in AF patients without visible LA structural change.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Impact of Right Ventricular Apical Pacing and Its Frequency on Left Atrial Function
Byung-Joo Choi, Kyoung-Im Cho, Seong-Man Kim, Yeo-Jeong Song, Hyeon-Gook Lee, Tae-Ik Kim
J Cardiovasc Ultrasound. 2012;20(1):42-48. doi: 10.4250/jcu.2012.20.1.42.
Reference
-
1. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995. 98:476–484.2. Allessie MA, Boyden PA, Camm AJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001. 103:769–777.3. van Wagoner DR, Nerbonne JM. Molecular basis of electrical remodeling in atrial fibrillation. J Mol Cell Cardiol. 2000. 32:1101–1117.4. Allessie MA. Atrial electrophysiological remodeling: another vicious circle? J Cardiovasc Electrophysiol. 1998. 9:1378–1393.5. Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997. 96:1686–1695.6. Schnee JM, Hsueh WA. Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc Res. 2000. 46:264–268.7. Ruiz-Ortega M, Lorenzo O, Ruperez M, et al. Role of the renin-angiotensin system in vascular diseases: expanding the field. Hypertension. 2001. 38:1382–1387.8. Willems R, Sipido KR, Holemans P, Ector H, van de Werf F, Heidbuchel H. Different patterns of angiotensin II and atrial natriuretic peptide secretion in a sheep model of atrial fibrillation. J Cardiovasc Electrophysiol. 2001. 12:1387–1392.9. Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. 1999. 100:376–380.10. Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias. Circulation. 2001. 104:2886–2891.11. Hwang SJ, Kim BJ, Shin HS, et al. Assessment of factors influencing plasma BNP level in patients with chronic atrial fibrillation and preserved left ventricular systolic function. Korean Circ J. 2005. 35:605–612.12. Hatle L, Sutherland GR. Regional myocardial function: a new approach. Eur Heart J. 2000. 21:1337–1357.13. Cho KI, Lee HG, Kim TI, et al. Quantitative assessment of left atrial function changes in patients with atrial fibrillation by tissue Doppler strain and 2-dimensional strain imaging. Korean Circ J. 2006. 36:786–793.14. Korinek J, Wang J, Sengupta PP, et al. Two-dimensional strain: a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr. 2005. 18:1247–1253.15. Barbier P, Alioto G, Guazzi MD. Left atrial function and ventricular filling in hypertensive patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 1994. 24:165–170.16. Sutherland GR, Hatle L, Rademakers FE, et al. Doppler Myocardial Imaging: A Textbook. 2003. Leuven, Belgium: Leuven University Press;99–107.17. Di Salvo G, Pacileo G, Del Giudice EM, et al. Atrial myocardial deformation properties in obese nonhypertensive children. J Am Soc Echocardiogr. 2008. 21:151–156.18. Thomas L, Levett K, Boyd A, Leung DY, Schiller NB, Ross DL. Changes in regional left atrial function with aging: evaluation by Doppler tissue imaging. Eur J Echocardiogr. 2003. 4:92–100.19. Di Salvo G, Caso P, Lo Piccolo R, et al. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: a color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation. 2005. 112:387–395.20. Hobbs WJ, van Gelder IC, Fitzpatrick AP, Crijns HJ, Garratt CJ. The role of atrial electrical remodeling in the progression of focal atrial ectopy to persistent atrial fibrillation. J Cardiovasc Electrophysiol. 1999. 10:866–870.21. Hwang GS, Kim YH, Lee HS, et al. Electrophysiologic properties of the atrium in patients with chronic and paroxysmal atrial fibrillation. Korean Circ J. 2000. 30:448–456.22. Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997. 96:1180–1184.23. Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999. 100:87–95.24. Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997. 59:551–571.25. Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation. 2000. 101:2612–2617.26. Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003. 41:2197–2204.27. Wazni OM, Martin DO, Marrouche NF, et al. Plasma B-type natriuretic peptide levels predict postoperative atrial fibrillation in patients undergoing cardiac surgery. Circulation. 2004. 110:124–127.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Influence of Electrical Cardioversion for Atrial Fibrillation on Left Atrial Appendage Function: A Transesophageal Echocardiography Study
- Relation between Atrial Fibrillation and Echocardiographic Size of Left Atrium
- Left Atrial Size in Hypertensive Patients with Atrial Fibrillation
- Quantitative Assessment of Left Atrial Functional Changes in Patients with Atrial Fibrillation by Tissue Doppler Strain and 2-Dimensional Strain Imaging
- Pathophysiology and Diagnosis in Atrial Fibrillation