Korean J Clin Neurophysiol.
2015 Jun;17(1):1-16. 10.14253/kjcn.2015.17.1.1.
Update of Therapeutic Clinical Trials for Amyotrophic Lateral Sclerosis
- Affiliations
-
- 1Department of Neurology, Dongguk University Ilsan Hospital, Goyang, Korea. nheekim8@hanmail.net
- KMID: 2225214
- DOI: http://doi.org/10.14253/kjcn.2015.17.1.1
Abstract
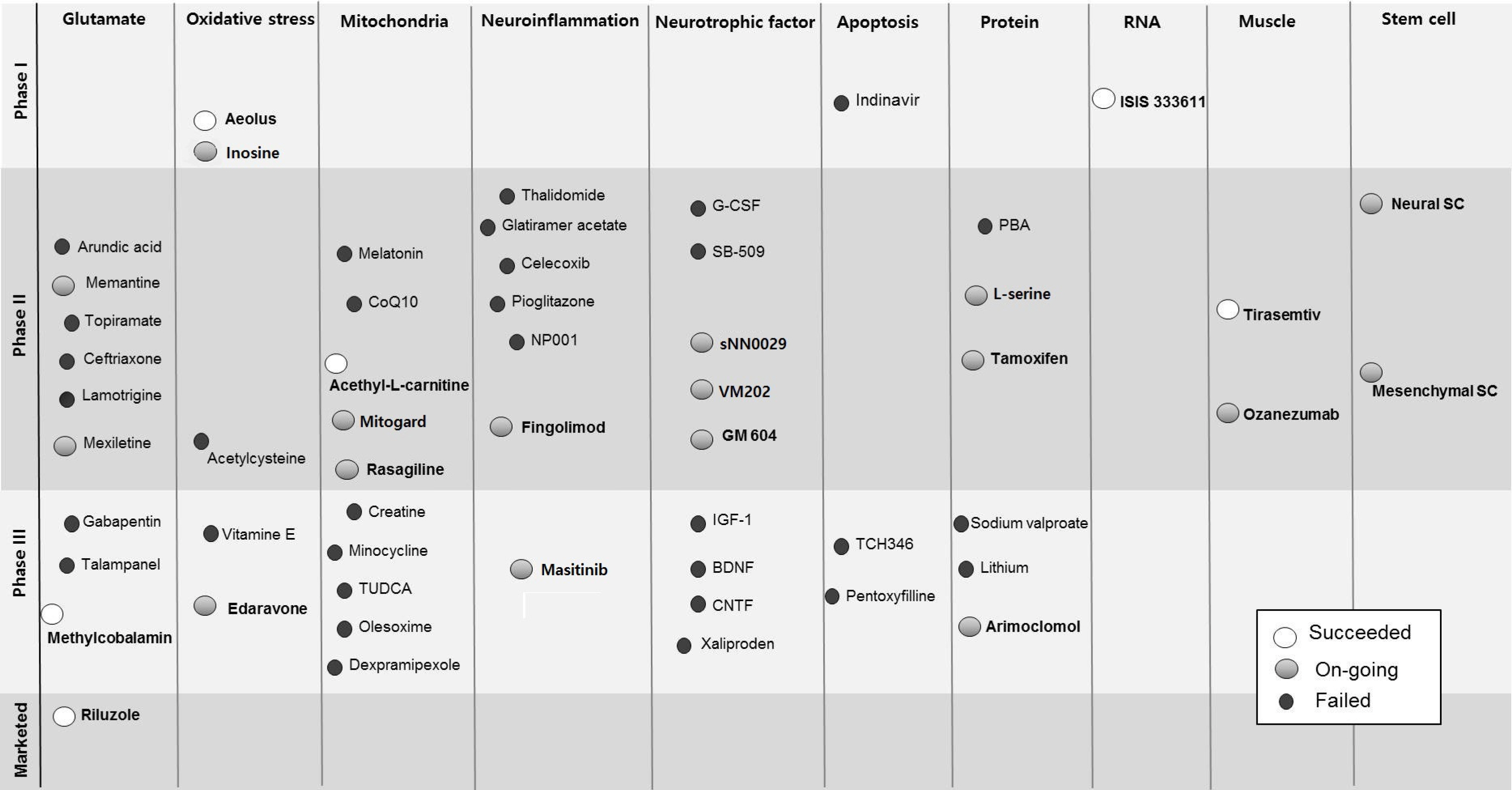
- Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that is characterized by progressive death of motor neurons in the cortex, brainstem, and spinal cord. Until now, many treatment strategies have been tested in ALS, but so far only Riluzole has shown efficacy of slightly slowing disease progression. The pathophysiological mechanisms underlying ALS are multifactorial, with a complex interaction between genetic factors and molecular pathways. Other motor neuron disease such as spinal muscular atrophy (SMA) and spinobulbar muscular atrophy (SBMA) are also progressive neurodegenerative disease with loss of motor neuron as ALS. This common thread of motor neuron loss has provided a target for the development of therapies for these motor neuron diseases. A better understanding of these pathogenic mechanisms and the potential pathological relationship between the various cellular processes have suggested novel therapeutic approaches, including stem cell and genetics-based strategies, providing hope for feasible treatment of ALS.
MeSH Terms
Figure
Reference
-
1.Morren JA., Galvez-Jimenez N. Current and prospective disease-modifying therapies for amyotrophic lateral sclerosis. Expert Opin Investig Drugs. 2012. 21:297–320.
Article2.Bensimon G., Lacomblez L., Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994. 330:585–591.3.Miller RG., Mitchell JD., Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2012. 3:CD001447.
Article4.Miller RG., Moore DH 2nd., Gelinas DF., Dronsky V., Mendoza M., Barohn RJ, et al. Phase III randomized trial of gabapentin in patients with amyotrophic lateral sclerosis. Neurology. 2001. 56:843–848.
Article5.Cudkowicz ME., Shefner JM., Schoenfeld DA., Brown RH Jr.., Johnson H., Qureshi M, et al. A randomized, placebo-controlled trial of topiramate in amyotrophic lateral sclerosis. Neurology. 2003. 61:456–464.
Article6.Ryberg H., Askmark H., Persson LI. A double-blind randomized clinical trial in amyotrophic lateral sclerosis using lamotrigine: effects on CSF glutamate, aspartate, branched-chain amino acid levels and clinical parameters. Acta Neurol Scand. 2003. 108:1–8.
Article7.Piepers S., Veldink JH., de Jong SW., van der Tweel I., van der Pol WL., Uijtendaal EV, et al. Randomized sequential trial of valproic acid in amyotrophic lateral sclerosis. Ann Neurol. 2009. 66:227–234.
Article8.ClinicalTrials.gov. Amyotrophic lateral sclerosis [Internet]. Bethesda (MD, US): U.S. National Institutes of Health;c2015. [cited 2015 Feb 9]. Available from:. https://clinicaltrials.gov/ct2/results?term=ALS+AND+Amyotrophic+Lateral+Sclerosis+%28ALS%29.9.Tateishi N., Mori T., Kagamiishi Y., Satoh S., Katsube N., Morikawa E, et al. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part II: suppression of as-trocytic activation by a novel agent (R)-(-)-2-propyloctanoic acid (ONO-2506) leads to mitigation of delayed infarct ex-pansion and early improvement of neurologic deficits. J Cereb Blood Flow Metab. 2002. 22:723–734.
Article10.de Carvalho M., Pinto S., Costa J., Evangelista T., Ohana B., Pinto A. A randomized, placebo-controlled trial of memantine for functional disability in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010. 11:456–460.
Article11.Goyal NA., Mozaffar T. Experimental trials in amyotrophic lateral sclerosis: a review of recently completed, ongoing and planned trials using existing and novel drugs. Expert Opin Investig Drugs. 2014. 23:1541–1551.
Article12.Pascuzzi RM., Shefner J., Chappell AS., Bjerke JS., Tamura R., Chaudhry V, et al. A phase II trial of talampanel in subjects with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010. 11:266–271.
Article13.Berry JD., Shefner JM., Conwit R., Schoenfeld D., Keroack M., Felsenstein D, et al. Design and initial results of a multi-phase randomized trial of ceftriaxone in amyotrophic lateral sclerosis. PLoS One. 2013. 8:e61177.
Article14.Kong Q., Chang LC., Takahashi K., Liu Q., Schulte DA., Lai L, et al. Small-molecule activator of glutamate transporter EAAT2 translation provides neuroprotection. J Clin Invest. 2014. 124:1255–1267.
Article15.Izumi Y., Kaji R. [Clinical trials of ultra-high-dose methyl-cobalamin in ALS]. Brain Nerve. 2007. 59:1141–1147.16.Desnuelle C., Dib M., Garrel C., Favier A. A double-blind, placebo-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. ALS riluzole-tocopherol Study Group. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001. 2:9–18.17.Orrell RW. AEOL-10150 (Aeolus). Curr Opin Investig Drugs. 2006. 7:70–80.18.Abe K., Itoyama Y., Sobue G., Tsuji S., Aoki M., Doyu M, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014. 15:610–617.
Article19.Yoshino H., Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study). Amyotroph Lateral Scler. 2006. 7:241–245.
Article20.ALSTDI homepage. Research center: Clinical Trials [Internet]. Cambridge, (MA, USA): ALS Therapy Development Institute;c2015. [cited 2015 Feb 9]. Available from:. http://www.als.net/ALS-Research/ALS-Clinical-Trials/.21.Louwerse ES., Weverling GJ., Bossuyt PM., Meyjes FE., de Jong JM. Randomized, double-blind, controlled trial of acetylcysteine in amyotrophic lateral sclerosis. Arch Neurol. 1995. 52:559–564.
Article22.Paganoni S., Zhang M., Quiroz Zarate A., Jaffa M., Yu H., Cudkowicz ME, et al. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J Neurol. 2012. 259:1923–1928.
Article23.Zinman L., Cudkowicz M. Emerging targets and treatments in amyotrophic lateral sclerosis. Lancet Neurol. 2011. 10:481–490.
Article24.Gordon PH., Moore DH., Miller RG., Florence JM., Verheijde JL., Doorish C, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007. 6:1045–1053.
Article25.Shefner JM., Cudkowicz ME., Schoenfeld D., Conrad T., Taft J., Chilton M, et al. A clinical trial of creatine in ALS. Neurology. 2004. 63:1656–1661.
Article26.Kaufmann P., Thompson JL., Levy G., Buchsbaum R., Shefner J., Krivickas LS, et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann Neurol. 2009. 66:235–244.
Article27.Cudkowicz M., Bozik ME., Ingersoll EW., Miller R., Mitsumoto H., Shefner J, et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med. 2011. 17:1652–1656.
Article28.Cudkowicz ME., van den Berg LH., Shefner JM., Mitsumoto H., Mora JS., Ludolph A, et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet Neurol. 2013. 12:1059–1067.
Article29.Lenglet T., Lacomblez L., Abitbol JL., Ludolph A., Mora JS., Robberecht W, et al. A phase II-III trial of olesoxime in subjects with amyotrophic lateral sclerosis. Eur J Neurol. 2014. 21:529–536.
Article30.Min JH., Hong YH., Sung JJ., Kim SM., Lee JB., Lee KW. Oral solubilized ursodeoxycholic acid therapy in amyotrophic lateral sclerosis: a randomized cross-over trial. J Korean Med Sci. 2012. 27:200–206.
Article31.Weishaupt JH., Bartels C., Polking E., Dietrich J., Rohde G., Poeggeler B, et al. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J Pineal Res. 2006. 41:313–323.
Article32.Keep M., Elmer E., Fong KS., Csiszar K. Intrathecal cyclosporin prolongs survival of late-stage ALS mice. Brain Res. 2001. 894:327–331.
Article33.Cudkowicz ME., Shefner JM., Schoenfeld DA., Zhang H., Andreasson KI., Rothstein JD, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006. 60:22–31.
Article34.Stommel EW., Cohen JA., Fadul CE., Cogbill CH., Graber DJ., Kingman L, et al. Efficacy of thalidomide for the treatment of amyotrophic lateral sclerosis: a phase II open label clinical trial. Amyotroph Lateral Scler. 2009. 10:393–404.
Article35.Meininger V., Drory VE., Leigh PN., Ludolph A., Robberecht W., Silani V. Glatiramer acetate has no impact on disease progression in ALS at 40 mg/day: a double-blind, randomized, multicentre, placebo-controlled trial. Amyotroph Lateral Scler. 2009. 10:378–383.36.Miller RG., Zhang R., Block G., Katz J., Barohn R., Kasarskis E, et al. NP001 regulation of macrophage activation markers in ALS: A phase I clinical and biomarker study. Amyotroph Lateral Scler Frontotemporal Degener. 2014. 15:601–609.
Article37.Dupuis L., Dengler R., Heneka MT., Meyer T., Zierz S., Kassubek J, et al. A randomized, double blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PLoS One. 2012. 7:e37885.
Article38.Sorenson EJ., Windbank AJ., Mandrekar JN., Bamlet WR., Appel SH., Armon C, et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2008. 71:1770–1775.
Article39.Hansen R., Saikali KG., Chou W., Russell AJ., Chen MM., Vijayakumar V, et al. Tirasemtiv amplifies skeletal muscle response to nerve activation in humans. Muscle Nerve. 2014. 50:925–931.
Article40.Meininger V., Bensimon G., Bradley WR., Brooks B., Douillet P., Eisen AA, et al. Efficacy and safety of xaliproden in amyotrophic lateral sclerosis: results of two phase III trials. Amyo-troph Lateral Scler Other Motor Neuron Disord. 2004. 5:107–117.
Article41.Scelsa SN., MacGowan DJ., Mitsumoto H., Imperato T., LeValley AJ., Liu MH, et al. A pilot, double-blind, placebo-controlled trial of indinavir in patients with ALS. Neurology. 2005. 64:1298–1300.
Article42.Miller R., Bradley W., Cudkowicz M., Hubble J., Meininger V., Mitsumoto H, et al. Phase II/III randomized trial of TCH346 in patients with ALS. Neurology. 2007. 69:776–784.
Article43.Meininger V., Asselain B., Guillet P., Leigh PN., Ludolph A., Lacomblez L, et al. Pentoxifylline in ALS: a double-blind, randomized, multicenter, placebo-controlled trial. Neurology. 2006. 66:88–92.
Article44.Cross AH., Naismith RT. Established and novel disease-modifying treatments in multiple sclerosis. J Intern Med. 2014. 275:350–363.
Article45.Cudkowicz ME., Andres PL., Macdonald SA., Bedlack RS., Choudry R., Brown RH Jr. . Phase 2 study of sodium phenylbutyrate in ALS. Amyotroph Lateral Scler. 2009. 10:99–106.
Article46.Kalmar B., Lu CH., Greensmith L. The role of heat shock proteins in Amyotrophic Lateral Sclerosis: The therapeutic potential of Arimoclomol. Pharmacol Ther. 2014. 141:40–54.
Article47.Cudkowicz ME., Shefner JM., Simpson E., Grasso D., Yu H., Zhang H, et al. Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis. Muscle Nerve. 2008. 38:837–844.
Article48.Aggarwal SP., Zinman L., Simpson E., McKinley J., Jackson KE., Pinto H, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010. 9:481–488.
Article49.Dunlop RA., Cox PA., Banack SA., Rodgers KJ. The non-protein amino acid BMAA is misincorporated into human proteins in place of L-serine causing protein misfolding and aggregation. PLoS One. 2013. 8:e75376.
Article50.Miller TM., Pestronk A., David W., Rothstein J., Simpson E., Appel SH, et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol. 2013. 12:435–442.51.Shefner J., Cedarbaum JM., Cudkowicz ME., Maragakis N., Lee J., Jones D, et al. Safety, tolerability and pharmacodynamics of a skeletal muscle activator in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012. 13:430–438.
Article52.Thomsen GM., Gowing G., Svendsen S., Svendsen CN. The past, present, and future of stem cell clinical trials for ALS. Exp Neurol. 2014. 262:127–137.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amyotrophic Lateral Sclerosis Associated With CADASIL
- Diagnosis and management of amyotrophic lateral sclerosis
- Syndrome of Progressive Bulbar Palsy in Amyotrophic Lateral Sclerosis: A Case Report
- Apraxia of Eyelid Closure and Motor Impersistence of Eyelid in a Patient with Amyotrophic Lateral Sclerosis
- Diagnosis and Therapeutic Strategies of Amyotrophic Lateral Sclerosis