Korean Circ J.
2011 Jan;41(1):46-50. 10.4070/kcj.2011.41.1.46.
Management of a Remnant Electrode in a Patient With Cardioverter-Defibrillator Infection After Refusal of Intravascular Electrode Removal
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine School of Medicine, Dankook University, Cheonan, Korea. mel_lee@dankook.ac.kr
- KMID: 2225155
- DOI: http://doi.org/10.4070/kcj.2011.41.1.46
Abstract
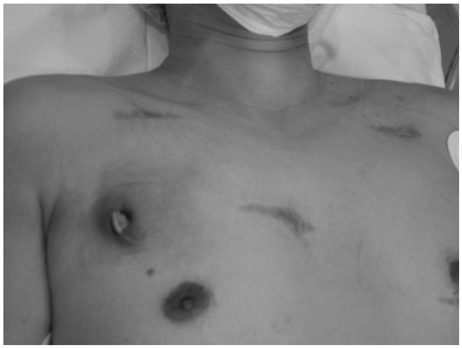
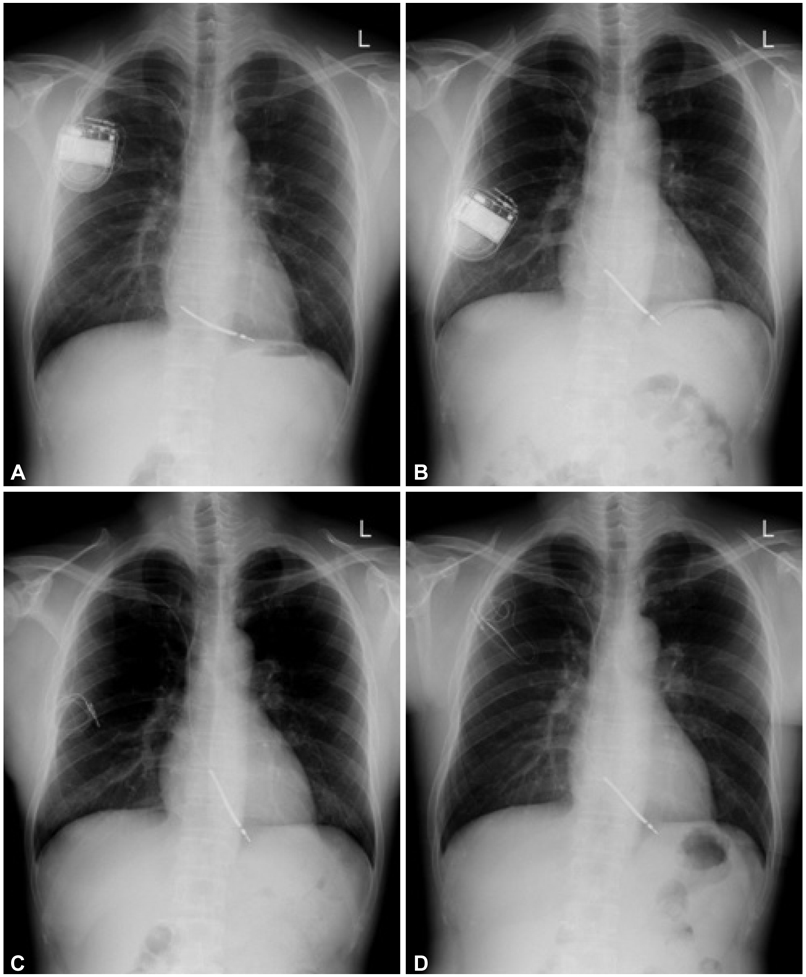
- Treatments of choice for cardiac implantable electronic device (CIED) infections are the removal of the entire CIED system, control of infection, and new device implantation. Occasionally, a complete CIED removal can not be performed for several reasons, such as very old age, severe comobidity, limited life expectancy, or refusal by a patient. We encountered a male patient who developed traumatic CIED infection five years after cardioverter-defibrillator implantation. An intravenous electrode could not be removed by a simple transvenous extraction procedure, and he refused surgical removal of the remnant electrode. After control of local infection, the tips of the electrode were separated and buried between muscles, and the wound was closed with a local flap. CIED infection did not recur for 12 months even without relying on long-term antimicrobial treatment.
MeSH Terms
Figure
Reference
-
1. Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000. 133:604–608.2. Sohail MR, Uslan DZ, Kahn AH, et al. Management and outcome of permanent and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007. 49:1851–1859.3. Love CJ, Wilkoff BL, Byrd CL, et al. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads; indications, facilities, training. Pacing Clin Electrophysiol. 2000. 23:544–551.4. Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003. 108:2015–2031.5. Gaynor SL, Zierer A, Lawton JS, Gleva MJ, Damiano RJ Jr, Moon MR. Laser assistance for extraction of chronically implanted endocardial leads: infectious versus noninfectious indications. Pacing Clin Electrophysiol. 2006. 29:1352–1358.6. Field ME, Jones SO, Epstein LM. How to select patients for lead extraction. Heart Rhythm. 2007. 4:978–985.7. Chua JD, Wilkoff BL, Lee I, et al. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000. 133:604–608.8. Sopeña B, Crespo M, Beiras X, et al. Individualized management of bacteraemia in patients with a permanent endocardial pacemaker. Clin Microbiol Infect. 2010. 16:274–280.9. Baddour LM. Long-term suppressive antimicrobial therapy for intravascular device-related infections. Am J Med Sci. 2001. 322:209–212.10. Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010. 121:458–477.11. Trappe HJ, Pfitzner P, Klein H, Wenzlaff P. Infections after cardioverter-defibrillator implantation: observations in 335 patients over 10 years. Br Heart J. 1995. 73:20–24.12. Lai KK, Fontecchio SA. Infections associated with implantable cardioverter-defibrillators placed transvenously and via thoracotomies: epidemiology, infection control, and management. Clin Infect Dis. 1998. 27:265–269.13. Gould PA, Krahn AD. Canadian Heart Rhythm Society Working Group on Device Advisories. Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006. 295:1907–1911.14. O'Nunain S, Perez I, Roelke M, et al. The treatment of patients with infected implantable cardioverter-defibrillator systems. J Thorac Cardiovasc Surg. 1997. 113:121–129.15. Sohail MR, Uslan DZ, Kahn AH, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc. 2008. 83:46–53.16. Baman TS, Gupta SK, Valle JA, Yamata E. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol. 2009. 2:129–134.17. de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009. 2:29–34.18. Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009. 95:715–720.19. Bloom H, Heeke B, Leon A, et al. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol. 2006. 29:142–145.20. Sohail MR, Uslan DZ, Khan AH, et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis. 2007. 45:166–173.21. Klug D, Balde M, Pavin D, et al. Risk factor related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007. 116:1349–1355.22. Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation. 1998. 97:1796–1801.23. Al-Khatib SM, Lucas FL, Jollis JG, Malenka DJ, Wennberg DE. The relation between patients' outcome and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiary. J Am Coll Cardiol. 2005. 46:1536–1540.24. Chamis AL, Perteson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation. 2001. 104:1029–1033.25. Kapa S, Hyberger L, Rea RF, Hayes DL. Complication risk with pulse generator change: implications when reacting to a device advisory or recall. Pacing Clin Electrophysiol. 2007. 30:730–733.26. Choi HS, Rhee MY, Choi YJ, et al. Long-term follow-up of the patients with permanent antibradycardia pacemaker. Korean Circ J. 1998. 28:768–773.27. Choi KJ, Lee CW, Kim JJ, Kim YH. Implantable cardioverter-defibrillator (ICD) therapy in a patient with the long QT syndrome. Korean Circ J. 1996. 26:1198–1203.28. Kim DH, Kim SY, Lee KH, et al. Follow-up of a group of patients with automatic implantable defibrillator. Korean Circ J. 2005. 35:69–83.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Removal of Entrapped Pacemaker Electrode: One Case Report
- Rupture of the Myocardium during the Removal of the Cardiac Pacemaker Electrode: A case report
- Transvenous Implantation of an Implantable Cardioverter Defibrillator in a Patient Who Had Undergone Tricuspid Valve Replacement
- Electroretinograms Using Skin Electrodes
- Comparison of Electroretinogram Waveforms Acquired Using Monopolar ERG-Jet Electrode and Bipolar Burian-Allen Electrode