Korean Circ J.
2011 Nov;41(11):677-680. 10.4070/kcj.2011.41.11.677.
Drug-Eluting Stent as an Option for Intractable In-Stent Coronary Restenosis
- Affiliations
-
- 1The Heart Center of Sapporo Higashi Tokushukai Hospital, Sapporo, Japan.
- 2The Heart Center of Chonnam National University Hospital, Gwangju, Korea. myungho@chollian.net
- KMID: 2225078
- DOI: http://doi.org/10.4070/kcj.2011.41.11.677
Abstract
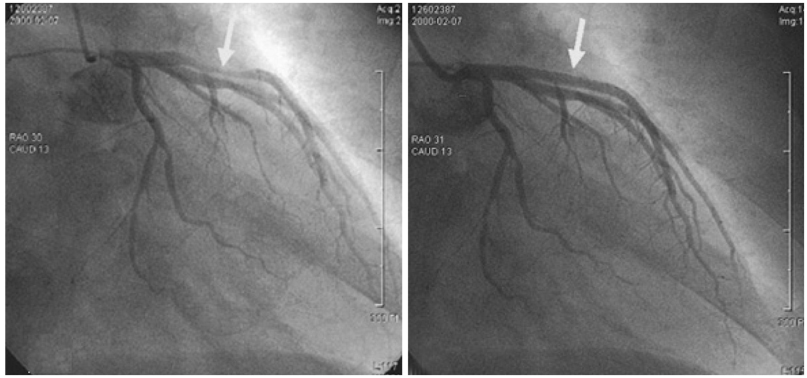
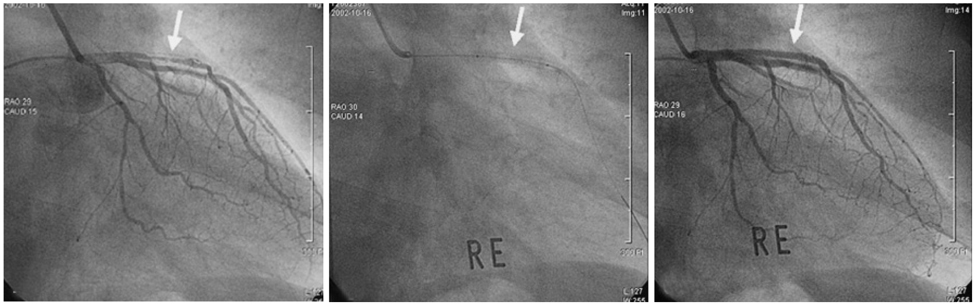
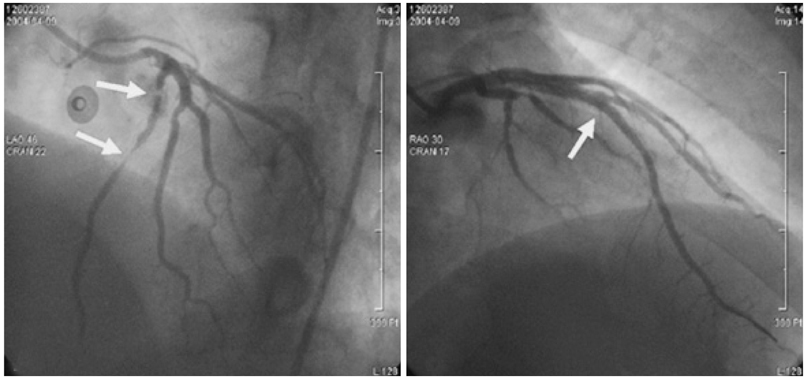
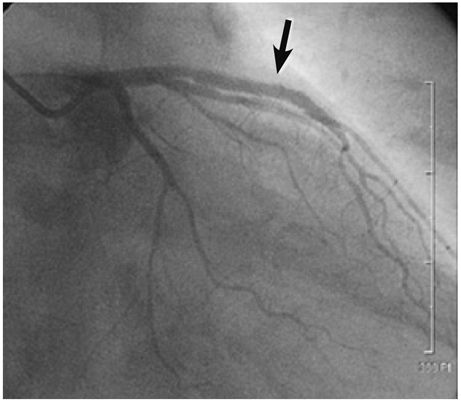
- A 51-year-old man was admitted due to an acute anterior ST-segment elevation myocardial infarction. After thrombolytic therapy using recombinant tissue plasminogen activator, stent implantation was performed from the proximal left anterior descending artery (LAD) to the mid LAD using a bare-metal stent (BMS). Since then, the patient suffered five repeated episodes of in-stent restenosis (ISR). At the first ISR, he was treated with plain old balloon angioplasty (POBA). At the second ISR, he was treated with brachytherapy, and at the third ISR, he was treated with POBA and one more BMS distal to the previously implanted stent. At the forth, only POBA was performed, and finally, at the fifth ISR, a sirolimus-eluting stent was implanted. Following that, the patient remained asymptomatic and follow-up coronary angiography showed no ISR.
MeSH Terms
Figure
Reference
-
1. Serruys PW, de Jaegere P, Kiemeneij F, et al. Benestent Study Group. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994. 331:489–495.2. Ahmed JM, Mintz GS, Weissman NJ, et al. Mechanism of lumen enlargement during intracoronary stent implantation: an intravascular ultrasound study. Circulation. 2000. 102:7–10.3. Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006. 151:1260–1264.4. Stone GW, Ellis SG, Colombo A, et al. Offsetting impact of thrombosis and restenosis on the occurrence of death and myocardial infarction after paclitaxel-eluting and bare metal stent implantation. Circulation. 2007. 115:2842–2847.5. Waksman R, Bhargava B, White L, et al. Intracoronary beta-radiation therapy inhibits recurrence of in-stent restenosis. Circulation. 2000. 101:1895–1898.6. Popma JJ, Suntharalingam M, Lansky AJ, et al. Randomized trial of 90Sr/90Y beta-radiation versus placebo control for treatment of in-stent restenosis. Circulation. 2002. 106:1090–1096.7. Stone GW, Ellis SG, O'Shaughnessy CD, et al. Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. JAMA. 2006. 295:1253–1263.8. Alfonso F, Pérez-Vizcayno MJ, Hernandez R, et al. A randomized comparison of sirolimus-eluting stent with balloon angioplasty in patients with in-stent restenosis: results of the restenosis intrastent: balloon angioplasty versus elective sirolimus-eluting stenting (RIBS-II) trial. J Am Coll Cardiol. 2006. 47:2152–2160.9. Fischman DL, Leon MB, Baim DS, et al. Stent Restenosis Study Investigators. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med. 1994. 331:496–501.10. Kastrati A, Mehilli J, Dirschinger J, et al. Restenosis after coronary placement of various stent types. Am J Cardiol. 2001. 87:34–39.11. Schühlen H, Kastrati A, Mehilli J, et al. Restenosis detected by routine angiographic follow-up and late mortality after coronary stent placement. Am Heart J. 2004. 147:317–322.12. Radke PW, Kaiser A, Frost C, Sigwart U. Outcome after treatment of coronary in-stent restenosis; results from a systematic review using meta-analysis techniques. Eur Heart J. 2003. 24:266–273.13. Hong YJ, Jeong MH. New methods of vascular brachytherapy for coronary stent restenosis. Korean Circ J. 2003. 33:967–976.14. Kim SH, Kim MH, Cha KS, et al. Effect of vascular brachytherapy using HolmiuM-166 liquid balloon system after cutting balloon angioplasty in patients with stent restenosis (BRAHMS Study). Korean Circ J. 2005. 35:591–596.15. Kastrati A, Mehilli J, von Beckerath N, et al. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005. 293:165–171.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug-Eluting Stent Strut Fracture as a Cause of Restenosis
- Systemic drug therapy and restenosis after drug-eluting stent implantation
- Risk of Stent Stenosis after Implanting a First-Generation Drug-Eluting Stent and Drug Balloon Angioplasty
- Drug-Eluting Stent Used to Treat a Case of Recurrent Right Coronary Artery In-Stent Restenoses often Accompanied by Acute Inferior Wall Myocardial Infarction
- Coronary Restenosis after Drug-Eluting Stent Implantation in Diabetic Patients