Korean Circ J.
2014 Sep;44(5):291-300. 10.4070/kcj.2014.44.5.291.
How to Achieve Complete and Permanent Pulmonary Vein Isolation without Complications
- Affiliations
-
- 1Central Utah Clinic-Cardiology, Utah Valley Regional Medical Center, Provo, UT, USA. chunhwang17@gmail.com
- 2Department of Cardiology, Dongsan Medical Center, Keimyung University, Daegu, Korea.
- KMID: 2223867
- DOI: http://doi.org/10.4070/kcj.2014.44.5.291
Abstract
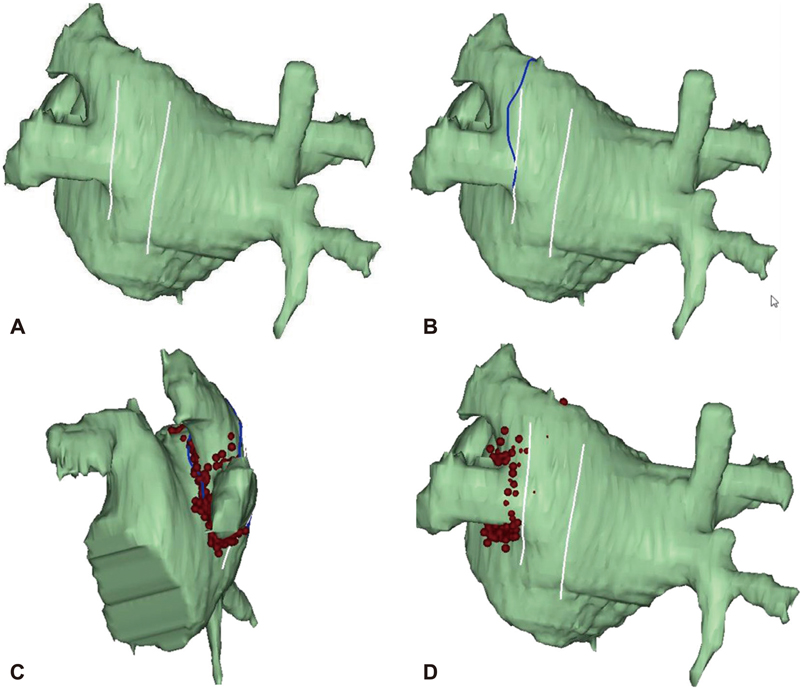
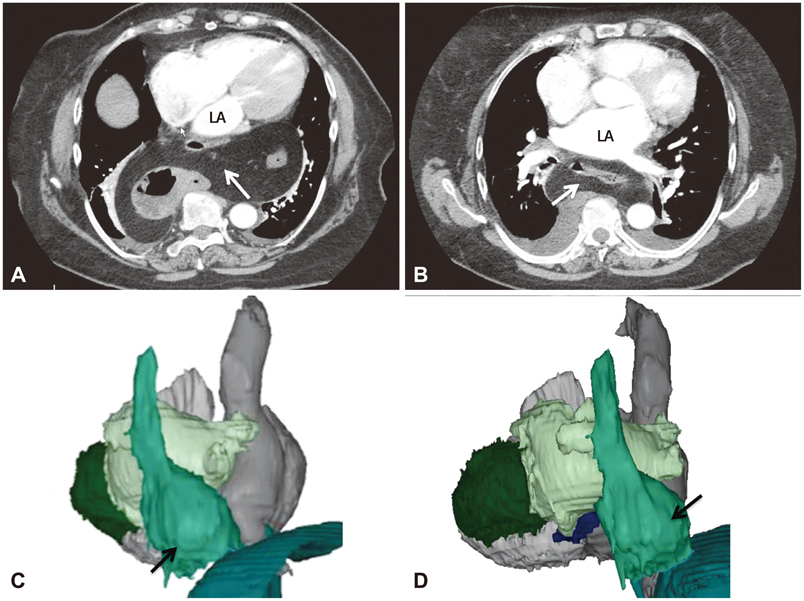
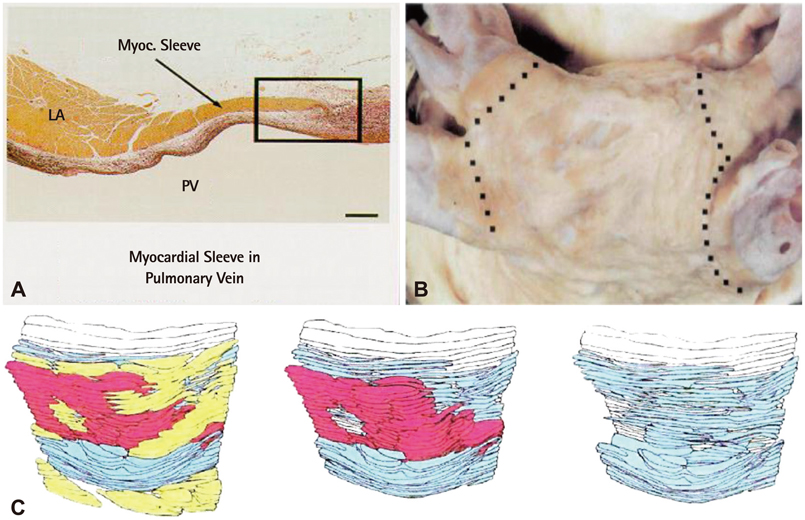
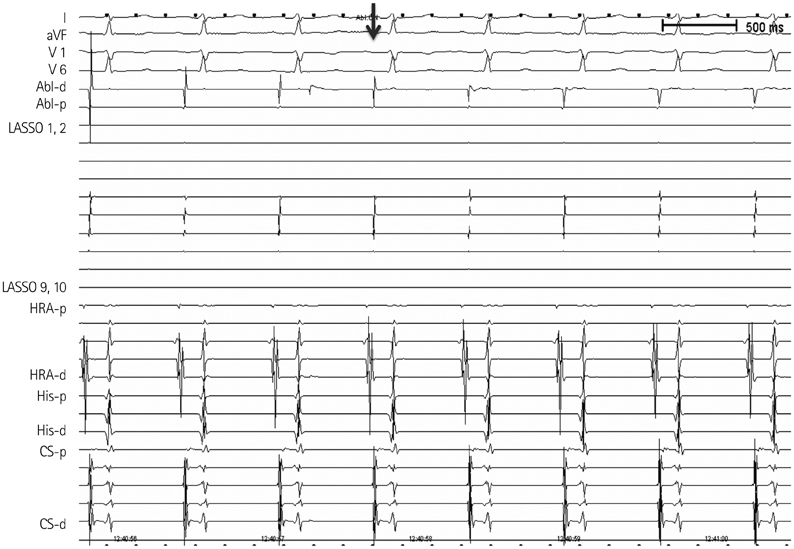
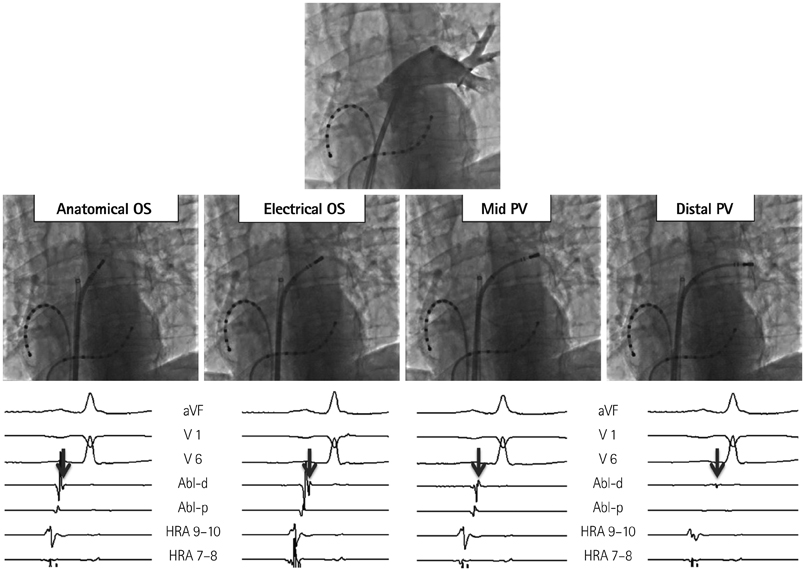
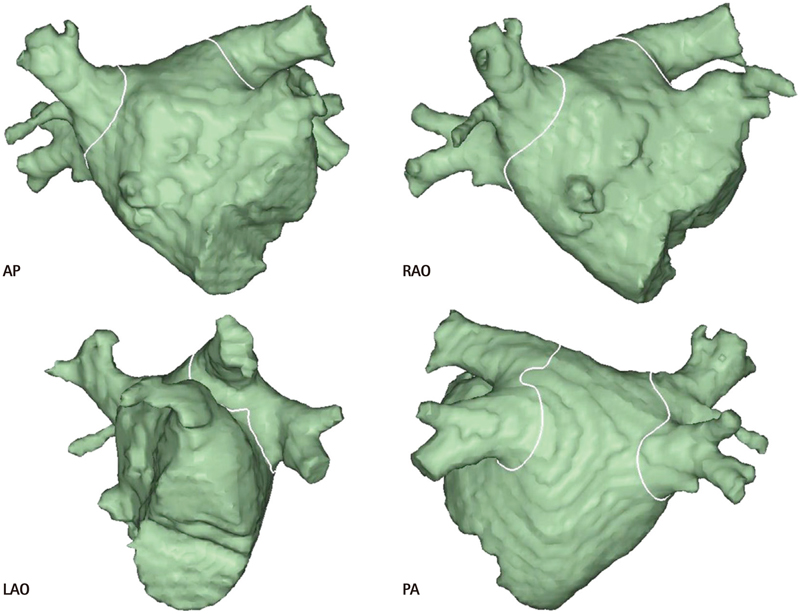
- The efficacy and safety of catheter ablation for the management of atrial fibrillation (AF) has been improved in recent years. Radiofrequency (RF) catheter ablation for maintaining sinus rhythm is superior to the current antiarrhythmic drug therapy in selected patients. Pulmonary vein isolation (PVI) is the cornerstone of various catheter ablation strategies. It is well recognized that pulmonary vein (PV) antrum contributes to the AF initiation and/or perpetuation. Since PV stenosis is a complication of ablation within a PV, the ablation site for PVI has shifted to the junction between the left atrium and the PV rather than the ostium of the PV. However, PV reconnection after ablation is the major cause of recurrence of AF. The recovery of PV conduction could be caused by anatomical variations such as the failure to produce complete transmural lesion or gaps at the ablation line due to the transient electrophysiologic effects from the RF ablation. In this review, we discussed several factors to be considered for the achievement of the best PVI, including clinical aspects and technical aspects.
MeSH Terms
Figure
Reference
-
1. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012; 9:632–696.2. Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009; 2:349–361.3. Piccini JP, Lopes RD, Kong MH, Hasselblad V, Jackson K, Al-Khatib SM. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol. 2009; 2:626–633.4. Parkash R, Tang AS, Sapp JL, Wells G. Approach to the catheter ablation technique of paroxysmal and persistent atrial fibrillation: a meta-analysis of the randomized controlled trials. J Cardiovasc Electrophysiol. 2011; 22:729–738.5. Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998; 339:659–666.6. Haïssaguerre M, Jaïs P, Shah DC, et al. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000; 101:1409–1417.7. Kalifa J, Jalife J, Zaitsev AV, et al. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003; 108:668–671.8. Kumagai K, Ogawa M, Noguchi H, Yasuda T, Nakashima H, Saku K. Electrophysiologic properties of pulmonary veins assessed using a multielectrode basket catheter. J Am Coll Cardiol. 2004; 43:2281–2289.9. Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005; 112:789–797.10. Lin YJ, Tsao HM, Chang SL, et al. Role of high dominant frequency sites in nonparoxysmal atrial fibrillation patients: insights from high-density frequency and fractionation mapping. Heart Rhythm. 2010; 7:1255–1262.11. Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010; 3:32–38.12. Arbelo E, Brugada J, Hindricks G, et al. ESC-EURObservational Research Programme: the Atrial Fibrillation Ablation Pilot Study, conducted by the European Heart Rhythm Association. Europace. 2012; 14:1094–1103.13. Hassink RJ, Aretz HT, Ruskin J, Keane D. Morphology of atrial myocardium in human pulmonary veins: a postmortem analysis in patients with and without atrial fibrillation. J Am Coll Cardiol. 2003; 42:1108–1114.14. Saito T, Waki K, Becker AE. Left atrial myocardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol. 2000; 11:888–894.15. Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006; 48:132–143.16. McGann CJ, Kholmovski EG, Oakes RS, et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008; 52:1263–1271.17. Kowalski M, Grimes MM, Perez FJ, et al. Histopathologic characterization of chronic radiofrequency ablation lesions for pulmonary vein isolation. J Am Coll Cardiol. 2012; 59:930–938.18. Ranjan R, Kato R, Zviman MM, et al. Gaps in the ablation line as a potential cause of recovery from electrical isolation and their visualization using MRI. Circ Arrhythm Electrophysiol. 2011; 4:279–286.19. Wittkampf FH, Hauer RN, Robles de Medina EO. Control of radiofrequency lesion size by power regulation. Circulation. 1989; 80:962–968.20. Jain MK, Wolf PD. Temperature-controlled and constant-power radio-frequency ablation: what affects lesion growth? IEEE Trans Biomed Eng. 1999; 46:1405–1412.21. Kautzner J, Neuzil P, Peichl P, et al. Contact force, force time integral and lesion continuity are critical to improve durable PV isolation: EFFICAS II results. Heart Rhythm. 2012; 9:S28.22. Cabrera JA, Ho SY, Climent V, Fuertes B, Murillo M, Sánchez-Quintana D. Morphological evidence of muscular connections between contiguous pulmonary venous orifices: relevance of the interpulmonary isthmus for catheter ablation in atrial fibrillation. Heart Rhythm. 2009; 6:1192–1198.23. Andrade JG, Pollak SJ, Monir G, et al. Pulmonary vein isolation using a pace-capture-guided versus an adenosine-guided approach: effect on dormant conduction and long-term freedom from recurrent atrial fibrillation--a prospective study. Circ Arrhythm Electrophysiol. 2013; 6:1103–1108.24. Sotomi Y, Inoue K, Ito N, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol. 2013; 111:552–556.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Levoatriocardinal Vein Combined with Pulmonary Venous Varix Mimicking Arteriovenous Malformations: A Case Report
- Individual Pulmonary Vein Atresia in Adults: Report of Two Cases
- The effect of empirical superior vena cava isolation during total thoracoscopic ablation in patients with persistent atrial fibrillation
- Controlled Atrial Fibrillation after Pulmonary Vein Stenting
- Pulmonary Vein Varix: A Case Report