J Rheum Dis.
2013 Feb;20(1):17-23. 10.4078/jrd.2013.20.1.17.
Duration of Prophylactic Therapy Affects the Frequency of Gout Flare
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea. Baekhj@gilhospital.com
- KMID: 2223044
- DOI: http://doi.org/10.4078/jrd.2013.20.1.17
Abstract
OBJECTIVE
To evaluate the effect of prophylactic therapy on gout flare during urate lowering treatment.
METHODS
We retrospectively examined the data derived from 59 patients who had been treated with allopurinol for more than six months after stopping prophylactic medication at our rheumatology clinic. Demographic data (age, sex, disease duration, tophi and comorbidity), clinical and laboratory features, including presence of gout flare during urate lowering treatment, dose of allopurinol, serum uric acid level and creatinine clearance at initiation and six months later, were collected. For the subgroup analysis, the same data were collected in 46 patients who had been followed up at one year after stopping prophylactic medication.
RESULTS
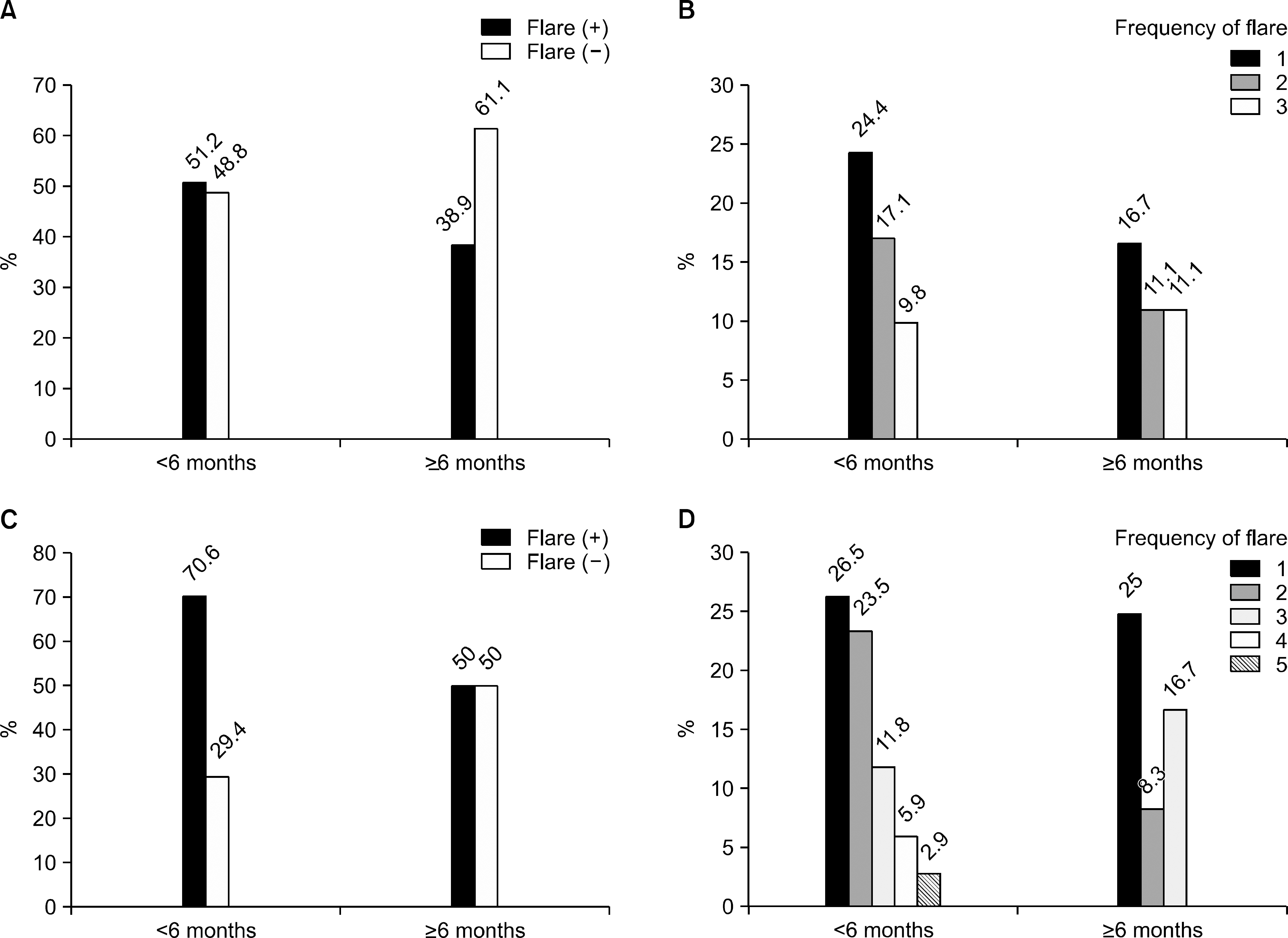
Twenty-eight patients among 59 (47.4%) had experienced at least 1 gouty attack during urate lowering therapy. The mean duration of prophylactic medication was not different between the flare group (3.8 months) and the non-flare group (5.9 months, p=0.617). Six months later, the mean serum uric acid level was 6.3 mg/dL (6.1 mg/dL vs. 6.5 mg/dL). According to the duration of prophylactic treatment (<6 months, > or =6 months), there were more frequent flares in the <6 months group than in the > or =6 months group (51.2% vs. 38.9% in the six month follow-up group, 70.6% vs. 50% in the one year follow-up group).
CONCLUSION
Prophylactic medication for more than six months could be a favorable factor for the prevention of recurrent gout flare during urate lowering treatment.
MeSH Terms
Figure
Reference
-
References
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011; 63:3136–41.
Article2. Annemans L, Spaepen E, Gaskin M, Bonnemaire M, Malier V, Gilbert T, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis. 2008; 67:960–6.
Article3. Keenan RT, O'Brien WR, Lee KH, Crittenden DB, Fisher MC, Goldfarb DS, et al. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med. 2011; 124:155–63.
Article4. Lee SJ, Hirsch JD, Terkeltaub R, Khanna D, Singh JA, Sarkin A, et al. Perceptions of disease and health-related quality of life among patients with gout. Rheumatology (Oxford). 2009; 48:582–6.
Article5. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007; 57:109–15.
Article6. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals followup study. Arch Intern Med. 2005; 165:742–8.7. Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011; 63:102–10.
Article8. Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Saag KG. Suboptimal physician adherence to quality indicators for the management of gout and asymptomatic hyperuricaemia: results from the UK General Practice Research Database (GPRD). Rheumatology (Oxford). 2005; 44:1038–42.
Article9. Son KM, Seo YI, Kim IJ, Bae YD, Jung YO, Cha MJ, et al. Adherence to uric acid lowering agent of gouty patients. J Korean Rheum Assoc. 2010; 17:162–7.
Article10. Spencer K, Carr A, Doherty M. Patient and provider bar-riers to effective management of gout in general practice: a qualitative study. Ann Rheum Dis. 2012; 71:1490–5.11. Emmerson BT. The management of gout. N Engl J Med. 1996; 334:445–51.
Article12. Wortmann RL. Effective management of gout: an analogy. Am J Med. 1998; 105:513–4.
Article13. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977; 20:895–900.
Article14. Gaffo AL, Schumacher HR, Saag KG, Taylor WJ, Dinnella J, Outman R, et al. Developing a provisional definition of flare in patients with established gout. Arthritis Rheum. 2012; 64:1508–17.
Article15. Taylor WJ, Shewchuk R, Saag KG, Schumacher HR Jr, Singh JA, Grainger R, et al. Toward a valid definition of gout flare: results of consensus exercises using Delphi methodology and cognitive mapping. Arthritis Rheum. 2009; 61:535–43.
Article16. Roddy E, Zhang W, Doherty M. Concordance of the management of chronic gout in a UK primary-care population with the EULAR gout recommendations. Ann Rheum Dis. 2007; 66:1311–5.
Article17. Harrold LR, Mazor KM, Velten S, Ockene IS, Yood RA. Patients and providers view gout differently: a qualitative study. Chronic Illn. 2010; 6:263–71.
Article18. Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010; 32:2386–97.
Article19. Zhang W, Doherty M, Pascual E, Bardin T, Barskova V, Conaghan P, et al. EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006; 65:1301–11.
Article20. Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, et al. British Society for Rheumatology and British Health Professionals in Rheumatology Standards, Guidelines and Audit Working Group (SGAWG). British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2007; 46:1372–4.
Article21. Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, et al. American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012; 64:1447–61.
Article22. Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004; 31:2429–32.23. Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010; 12:R63.
Article24. Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005; 353:2450–61.
Article25. Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004; 51:321–5.
Article26. Lim AY, Shen L, Tan CH, Lateef A, Lau TC, Teng GG. Achieving treat to target in gout: a clinical practice improvement project. Scand J Rheumatol. 2012; 41:450–7.
Article27. Reinders MK, Haagsma C, Jansen TL, van Roon EN, Delsing J, van de Laar MA, et al. A randomised controlled trial on the efficacy and tolerability with dose es-calation of allopurinol 300–600 mg/day versus benzbromarone 100–200 mg/day in patients with gout. Ann Rheum Dis. 2009; 68:892–7.
Article28. Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006; 47:51–9.
Article29. Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010; 5:1388–93.
Article30. Jo SY, Park YB, Lee CH. The Effect of hypouricemic treatment on the renal function in patients with gout. J Rheum Dis. 2011; 18:26–31.
Article31. Stamp LK, O'Donnell JL, Zhang M, James J, Frampton C, Barclay ML, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011; 63:412–21.
Article32. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012; 64:1431–46.
Article