J Rheum Dis.
2014 Dec;21(6):326-330. 10.4078/jrd.2014.21.6.326.
Leflunomide-induced Toxic Epidermal Necrolysis in a Patient with Rheumatoid Arthritis
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, College of Medicine, Korea University, Seoul, Korea. csjmd@hotmail.com
- KMID: 2222939
- DOI: http://doi.org/10.4078/jrd.2014.21.6.326
Abstract
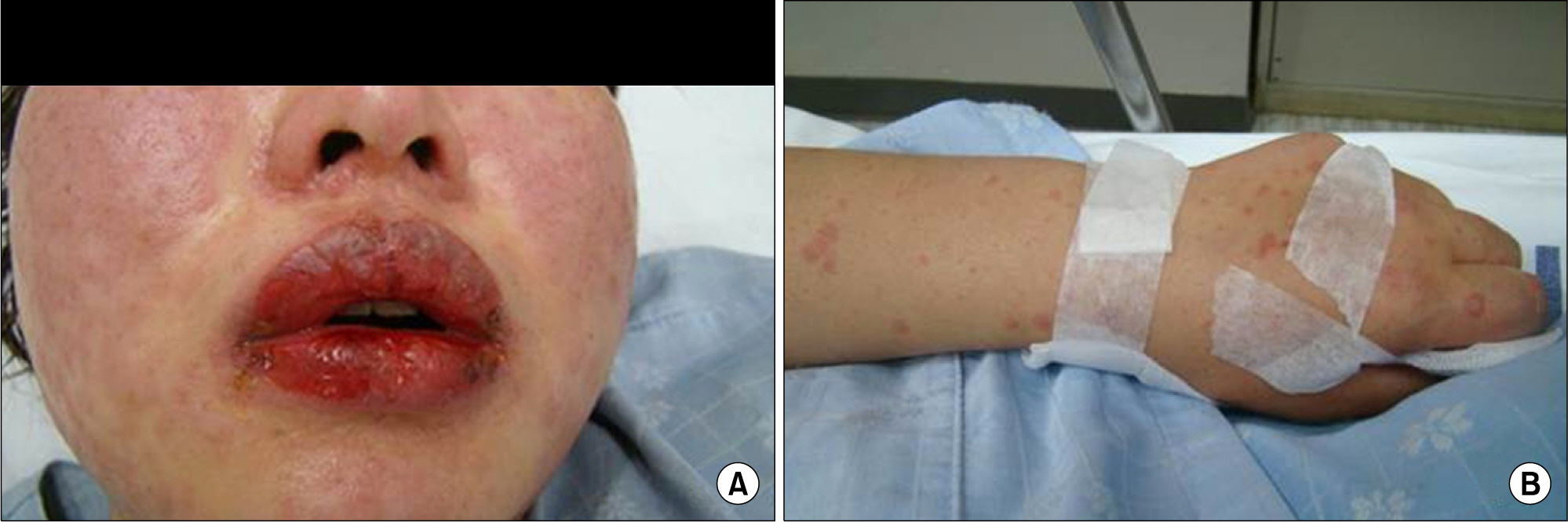
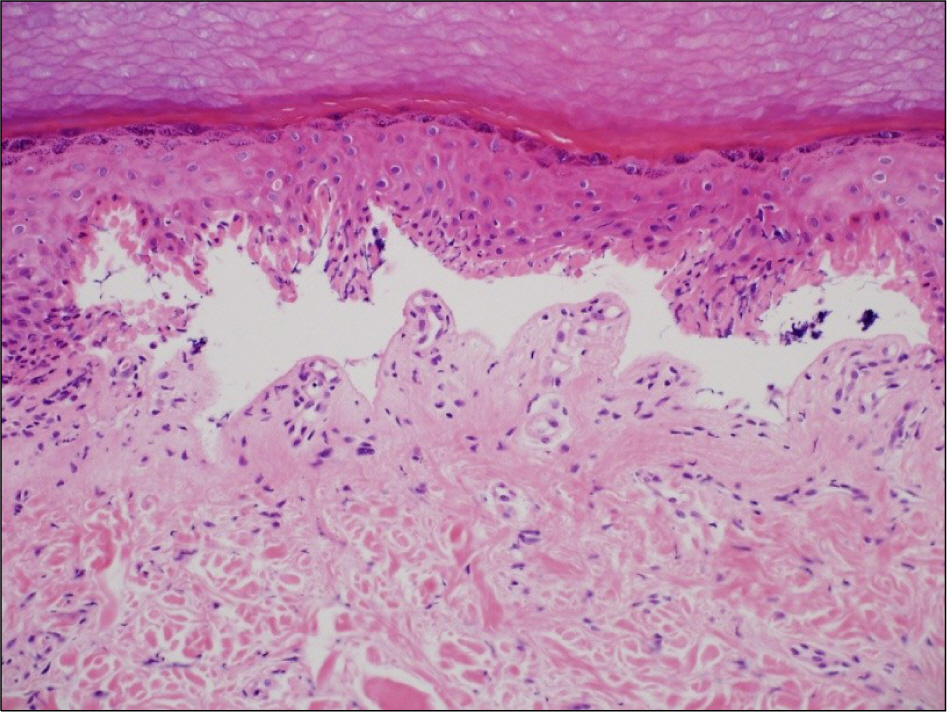
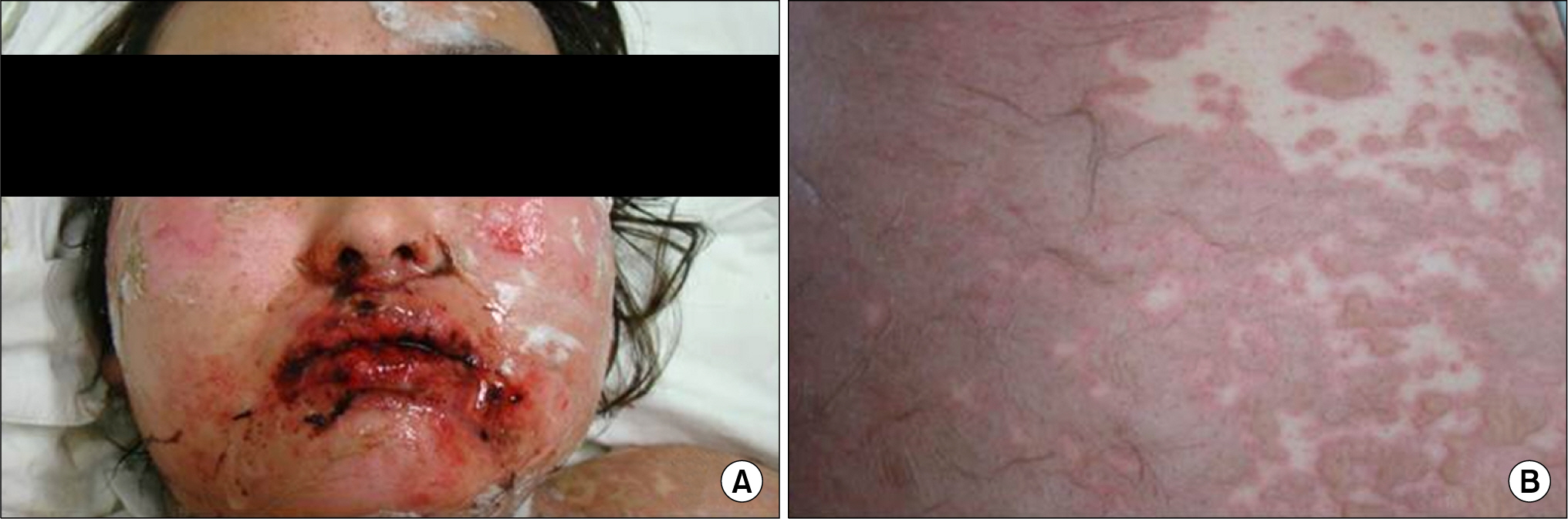
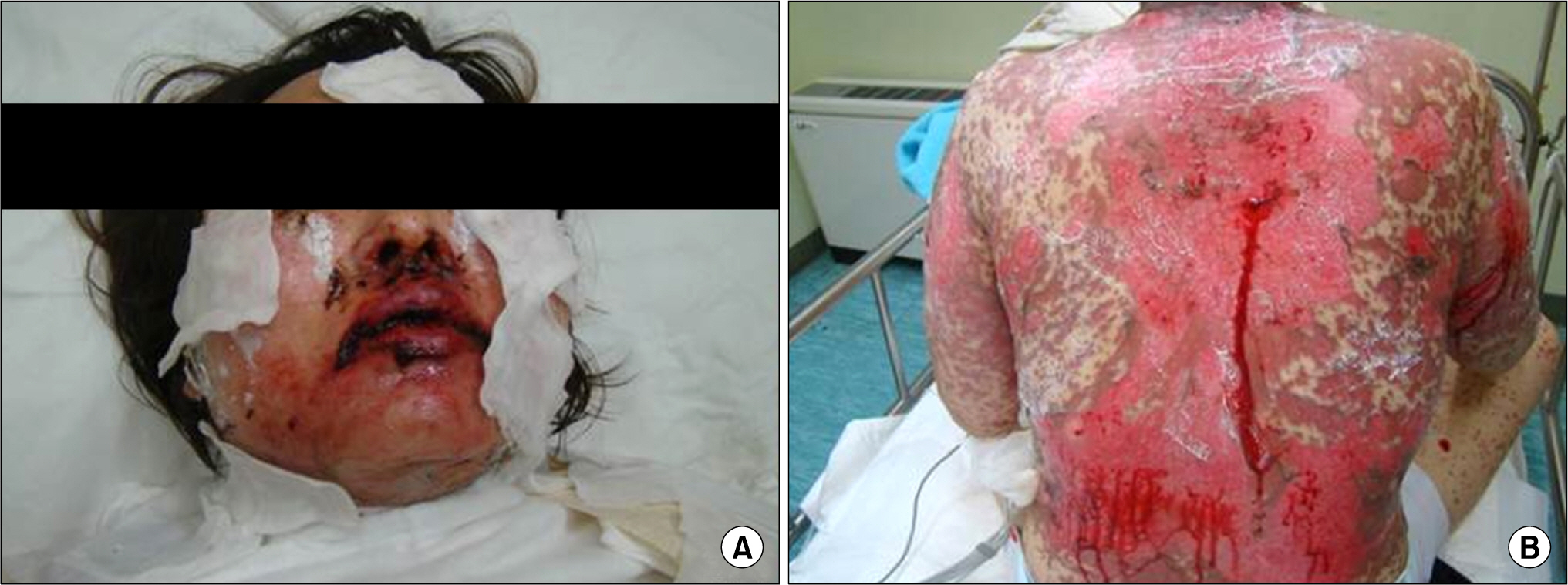
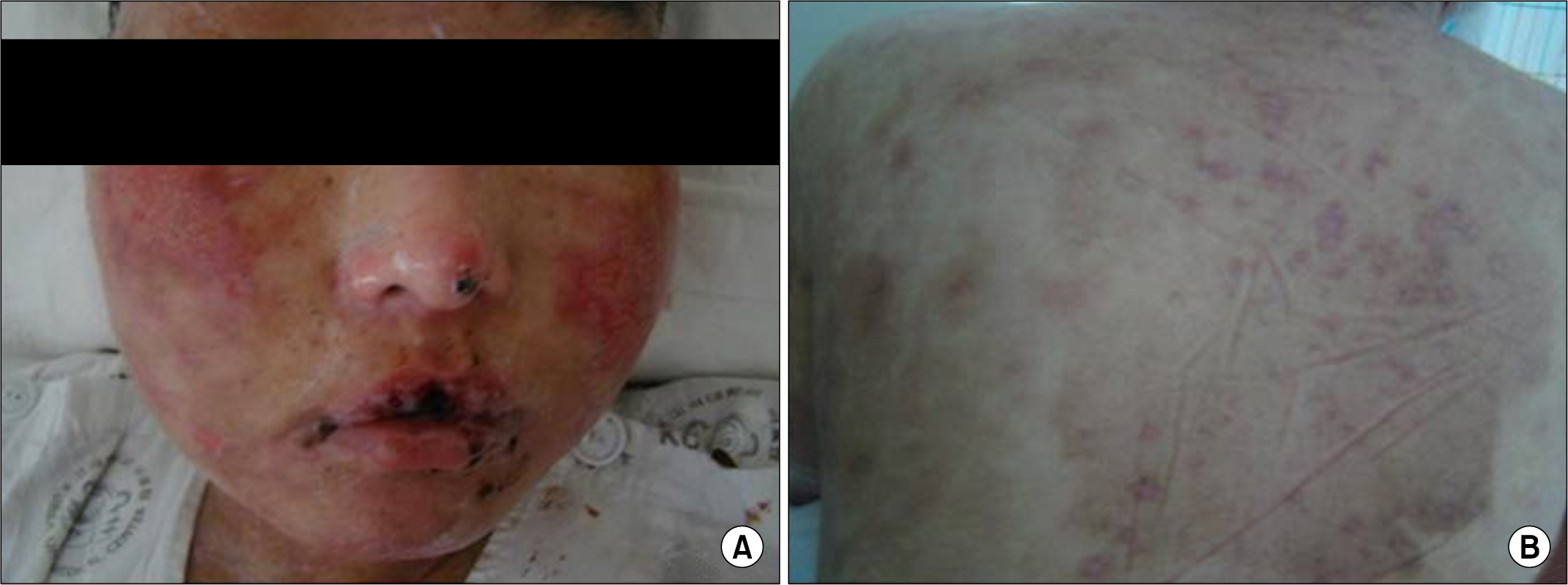
- Leflunomide was licensed for the treatment of rheumatoid arthritis in 1998 and has been available in Korea since 2003. Allergic cutaneous reactions (rash, purpura) are common (<10%) side effects of leflunomide, but severe cases such as Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) are rarely reported. There has not been a report of SJS or TEN induced by leflunomide in Korea. Here we report a case of leflunomide-induced TEN in a patient with rheumatoid arthritis. Leflunomide was discontinued, and the TEN was treated with methylprednisolone, cholestyramine and immunoglobulin. The skin lesion eventually resolved over four weeks with residual post-inflammatory hyperpigmentation.
MeSH Terms
Figure
Reference
-
1. van Riel PL, Smolen JS, Emery P, Kalden JR, Dougados M, Strand CV, et al. Leflunomide: a manageable safety profile. J Rheumatol Suppl. 2004; 71:21–4.2. Osiri M, Shea B, Robinson V, Suarez-Almazor M, Strand V, Tugwell P, et al. Leflunomide for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. 2003; 30:1182–90.3. Smolen JS, Emery P. Efficacy and safety of leflunomide in active rheumatoid arthritis. Rheumatology (Oxford). 2000; 39(Suppl 1):48–56.
Article4. Schmutz JL, Barbaud A, Tréchot P. Leflunomide and Lyell syndrome. Ann Dermatol Venereol. 2009; 136:395.5. Hassikou H, El Haouri M, Tabache F, Baaj M, Safi S, Hadri L. Leflunomide-induced toxic epidermal necrolysis in a patient with rheumatoid arthritis. Joint Bone Spine. 2008; 75:597–9.
Article6. El Aïdli S, Salouage I, Kastalli S, Srairi S, Daghfous R, Loueslati MH, et al. Leflunomide-induced toxic epidermal necrolysis. Therapie. 2008; 63:157–8.7. Teraki Y, Hitomi K, Sato Y, Hamamatsu Y, Izaki S. Leflunomide-induced toxic epidermal necrolysis. Int J Dermatol. 2006; 45:1370–1.
Article8. Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed.p. 325. Salt Lake City: Elsevier;2012.9. Kim HW, Park HC, Kim JE, Ko JY, Ro YS. Drug hypersensitivity syndrome induced by leflunomide. Korean J Dermatol. 2013; 51:226–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Carbamazepine- Induced- Toxic Epidermal Necrolysis
- A Case of Peripheral Neuropathy in a Patient with Rheumatoid Arthritis Treated with Leflunomide
- A Case of Toxic Epidermal Necrolysis Due to Contact of Paraquat(Gramoxone(R))
- A Case of Complete Vaginal Ostruction Following Toxic Epidermal Necrolysis
- A Case of Toxic Epidermal Necrolysis and Review of Literatures