J Korean Ophthalmol Soc.
2014 Feb;55(2):216-221. 10.3341/jkos.2014.55.2.216.
The Effect of Bevacizumab on Retinal Vessel Diameter, Intraocular Pressure, Retinal Nerve Fiber Layer and the Optic Disc
- Affiliations
-
- 1Department of Ophthalmology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea. hhiatus@gmail.com
- KMID: 2218414
- DOI: http://doi.org/10.3341/jkos.2014.55.2.216
Abstract
- PURPOSE
To evaluate the effects of a single intravitreal bevacizumab injection on retinal vessel diameter, intraocular pressure (IOP), retinal nerve fiber layer (RNFL) thickness and the optic disc in patients with diabetic macular edema (DME).
METHODS
In this retrospective study, 63 eyes with DME were included. All patients received an intravitreal injection of 1.25 mg bevacizumab. We reviewed retinal vessel diameter, IOP RNFL thickness and vertical cup-to-disc (C/D) ratios at the baseline and 7 days, 1 month, 3 months and 6 months after injection. The diameter of the central retinal arteries and veins were measured using retinal photographs. The central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE) were calculated using the revised Parr-Hubbard formula. RNFL thickness was obtained using optical coherence tomography. The vertical C/D ratio of the optic disc was evaluated using stereoscopic optic disc photography.
RESULTS
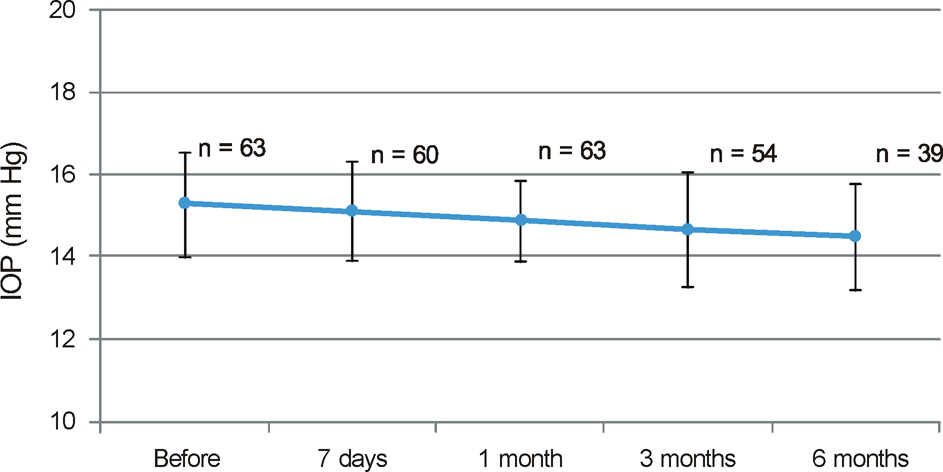
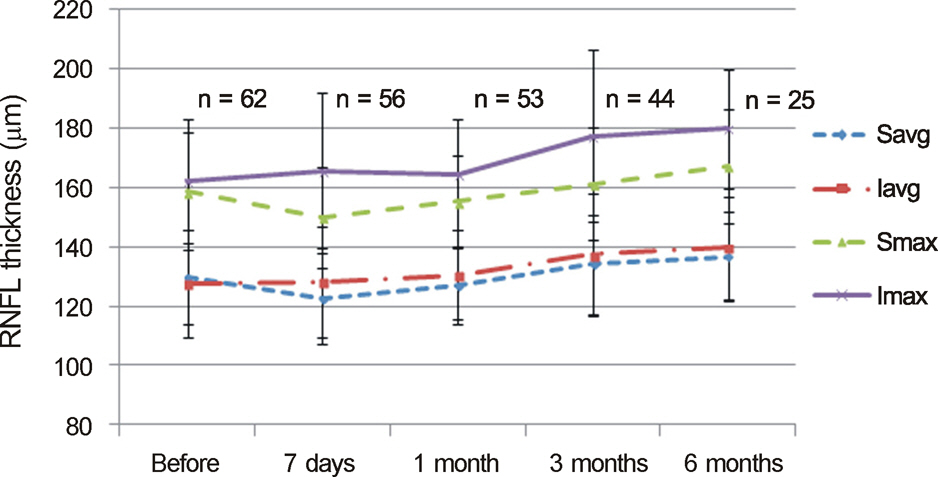
After bevacizumab injection, the CRAE significantly decreased at 7 days and 1 month postoperatively compared to baseline (p < 0.001 and p = 0.036, respectively). However, the changes in the CRAE at 3 months and 6 months were not statistically significant (p = 0.992 and p = 0.342, respectively). There were no statistically significant changes in the CRVE, mean IOP, RNFL thickness and vertical C/D ratios of the optic disc.
CONCLUSIONS
A single intravitreal bevacizumab injection transiently decreased the diameters of central retinal arterioles, but induced no significant changes in central venular diameter, mean IOP, RNFL thickness or vertical C/D ratios of the optic disc.
MeSH Terms
Figure
Reference
-
References
1. Andreoli CM, Miller JW.Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007; 18:502–8.
Article2. Chong V.Biological, preclinical and clinical characteristics of in-hibitors of vascular endothelial growth factors. Ophthalmologica. 2012; 227(Suppl 1):2–10.
Article3. Caldwell RB, Bartoli M, Behzadian MA. . Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mecha-nisms and treatment perspectives. Diabetes Metab Res Rev. 2003; 19:442–55.
Article4. Harris A, Ciulla TA, Chung HS, Martin B.Regulation of retinal and optic nerve blood flow. Arch Ophthalmol. 1998; 116:1491–5.
Article5. Tilton RG, Chang KC, LeJeune WS. . Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by in-travenous VEGF. Invest Ophthalmol Vis Sci. 1999; 40:689–96.6. Nishijima K, Ng YS, Zhong L. . Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuro-protectant during the adaptive response to ischemic injury. Am J Pathol. 2007; 171:53–67.
Article7. Drance SM, Sweeney VP, Morgan RW, Feldman F.Studies of fac-tors involved in the production of low tension glaucoma. Arch Ophthalmol. 1973; 89:457–65.
Article8. Mitchell P, Leung H, Wang JJ. . Retinal vessel diameter and open-angle glaucoma: the Blue Mountains Eye Study. Ophthalmology. 2005; 112:245–50.9. Chen SD, Wang L, Zhang XL.Neuroprotection in glaucoma: pres-ent and future. Chin Med J (Engl). 2013; 126:1567–77.10. Weinreb RN.Glaucoma neuroprotection: What is it? Why is it needed? Can J Ophthalmol. 2007; 42:396–8.
Article11. Bakri SJ, Snyder MR, Reid JM. . Pharmacokinetics of intra-vitreal bevacizumab (Avastin). Ophthalmology. 2007; 114:855–9.
Article12. Krohne TU, Liu Z, Holz FG, Meyer CH.Intraocular pharmacoki-netics of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol. 2012; 154:682–6.e2.
Article13. Patel RD, Momi RS, Hariprasad SM.Review of ranibizumab trials for neovascular age-related macular degeneration. Semin Ophthalmol. 2011; 26:372–9.
Article14. Nicholson BP, Schachat AP.A review of clinical trials of an-ti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010; 248:915–30.
Article15. van der Reis MI, La Heij EC, De Jong-Hesse Y. . A systematic review of the adverse events of intravitreal anti-vascular endothe-lial growth factor injections. Retina. 2011; 31:1449–69.
Article16. Mendrinos E, Mangioris G, Papadopoulou DN. . Retinal vessel analyzer measurements of the effect of panretinal photocoagulation on the retinal arteriolar diameter in diabetic retinopathy. Retina. 2010; 30:555–61.
Article17. Chang M, Yoo C, Kim SW, Kim YY.Retinal vessel diameter, reti-nal nerve fiber layer thickness, and intraocular pressure in korean patients with normal-tension glaucoma. Am J Ophthalmol. 2011; 151:100–5.e1.
Article18. Knudtson MD, Lee KE, Hubbard LD. . Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003; 27:143–9.
Article19. Foxton RH, Finkelstein A, Vijay S. . VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am J Pathol. 2013; 182:1379–90.
Article20. Papadopoulou DN, Mendrinos E, Mangioris G. . Intravitreal ranibizumab may induce retinal arteriolar vasoconstriction in patients with neovascular age-related macular degeneration. Ophthalmology. 2009; 116:1755–61.
Article21. Tatlipinar S, Dinç UA, Yenerel NM, Görgün E.Short-term effects of a single intravitreal bevacizumab injection on retinal vessel calibre. Clin Exp Optom. 2012; 95:94–8.
Article22. Jonas JB, Nguyen XN, Naumann GO.Parapapillary retinal vessel diameter in normal and glaucoma eyes. I. Morphometric data. Invest Ophthalmol Vis Sci. 1989; 30:1599–603.23. Rader J, Feuer WJ, Anderson DR.Peripapillary vasoconstriction in the glaucomas and the anterior ischemic optic neuropathies. Am J Ophthalmol. 1994; 117:72–80.
Article24. Rankin SJ, Drance SM.Peripapillary focal retinal arteriolar nar-rowing in open angle glaucoma. J Glaucoma. 1996; 5:22–8.
Article25. Kahook MY, Kimura AE, Wong LJ. . Sustained elevation in in-traocular pressure associated with intravitreal bevacizumab injections. Ophthalmic Surg Lasers Imaging. 2009; 40:293–5.
Article26. Bakri SJ, Pulido JS, McCannel CA. . Immediate intraocular pressure changes following intravitreal injections of triamcinolone, pegaptanib, and bevacizumab. Eye (Lond). 2009; 23:181–5.
Article27. Good TJ, Kimura AE, Mandava N, Kahook MY.Sustained ele-vation of intraocular pressure after intravitreal injections of an-ti-VEGF agents. Br J Ophthalmol. 2011; 95:1111–4.
Article28. Hoang QV, Tsuang AJ, Gelman R. . Clinical predictors of sus-tained intraocular pressure elevation due to intravitreal anti-vas-cular endothelial growth factor therapy. Retina. 2013; 33:179–87.
Article29. Wehrli SJ, Tawse K, Levin MH. . A lack of delayed intraocular pressure elevation in patients treated with intravitreal injection of bevacizumab and ranibizumab. Retina. 2012; 32:1295–301.
Article30. Horsley MB, Mandava N, Maycotte MA, Kahook MY.Retinal nerve fiber layer thickness in patients receiving chronic anti-vas-cular endothelial growth factor therapy. Am J Ophthalmol. 2010; 150:558–61.e1.
Article31. Seth RK, Salim S, Shields MB, Adelman RA.Assessment of optic nerve cup-to-disk ratio changes in patients receiving multiple intra-vitreal injections of antivascular endothelial growth factor agents. Retina. 2009; 29:956–9.
Article32. Martinez-de-la-Casa JM, Ruiz-Calvo A, Saenz-Frances F. . Retinal nerve fiber layer thickness changes in patients with age-re-lated macular degeneration treated with intravitreal ranibizumab. Invest Ophthalmol Vis Sci. 2012; 53:6214–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optic Disc Pit with Peripapillary Retinoschisis Presenting as a Localized Retinal Nerve Fiber Layer Defect
- Aplasia of the Optic Nerve
- Changes in the Retinal Nerve Fiber Layer after Intravitreal Injections of Bevacizumab in Glaucoma Patients
- Retinal Nerve Fiber Layer Defect Associated with Astrocytic Hamartoma in a Patient with Tuberous Sclerosis
- A Case of Partial Evulsion of Optic Nerve Associated with Retinal Tear