J Korean Ophthalmol Soc.
2013 May;54(5):745-751. 10.3341/jkos.2013.54.5.745.
The Study of Ocular Side Effects after the Use of Anti-Glaucoma Topical Medication
- Affiliations
-
- 1Department of Ophthalmology, Chonnam National University Medical School, Gwangju, Korea. exo70@naver.com
- KMID: 2217040
- DOI: http://doi.org/10.3341/jkos.2013.54.5.745
Abstract
- PURPOSE
To investigate the influence of number and type of side effects as well as treatment period after the use of anti-glaucoma eye drops in patients with glaucoma.
METHODS
A survey was conducted regarding the severity of ocular side effects after the use of anti-glaucoma eye drops in 528 patients with glaucoma. Side effects after using the eye drops included irritability, blurred vision, foreign body sensation, hyperemia, pinpricking sensation, itching, discomfort, pain, tearing, dry eye, and pigmentation. We analyzed the results to investigate the influence of number and type of side effects as well as treatment period.
RESULTS
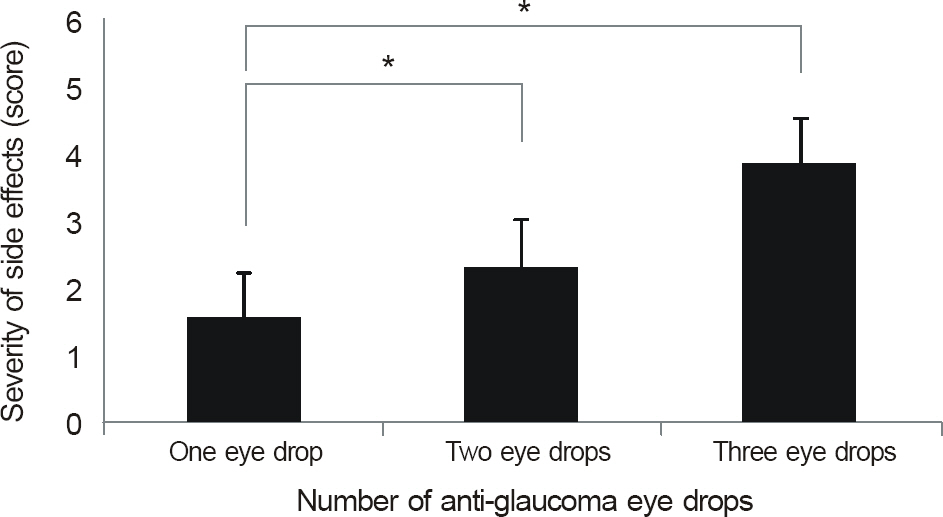
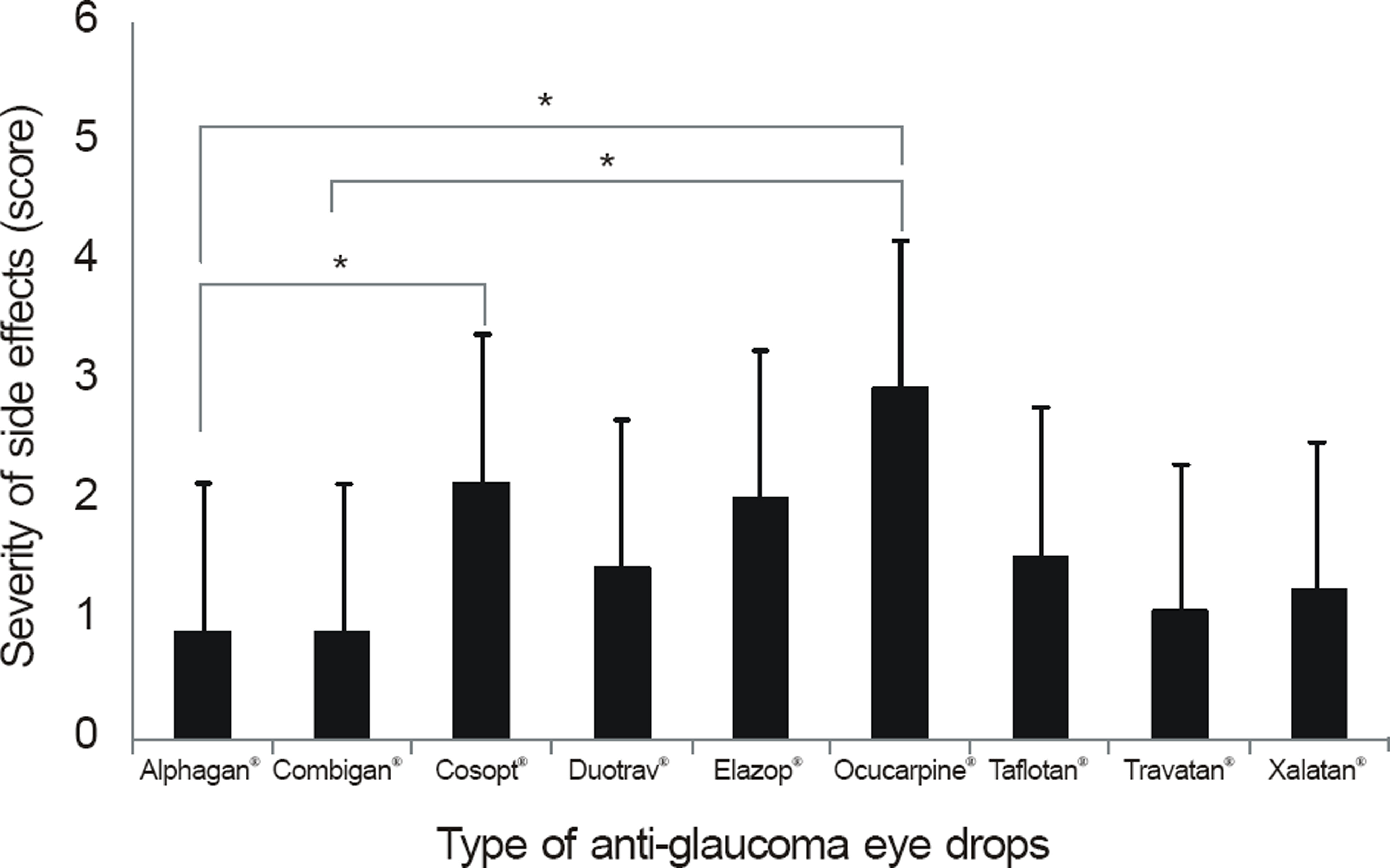
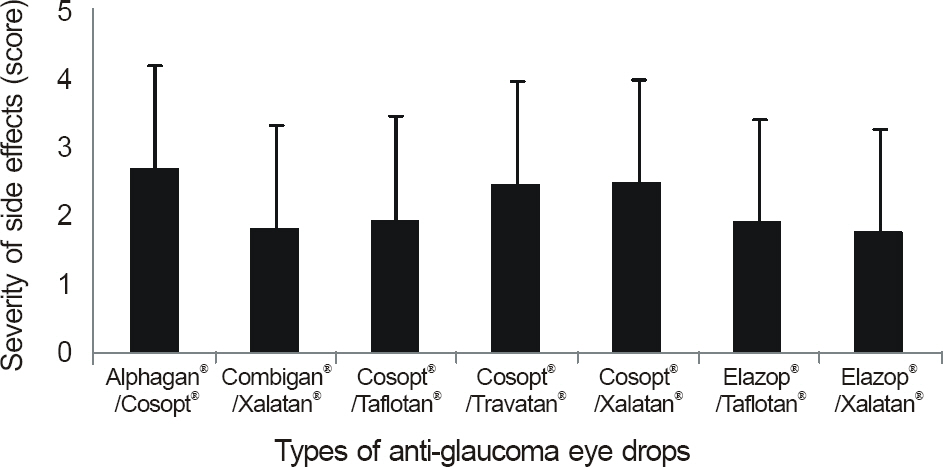
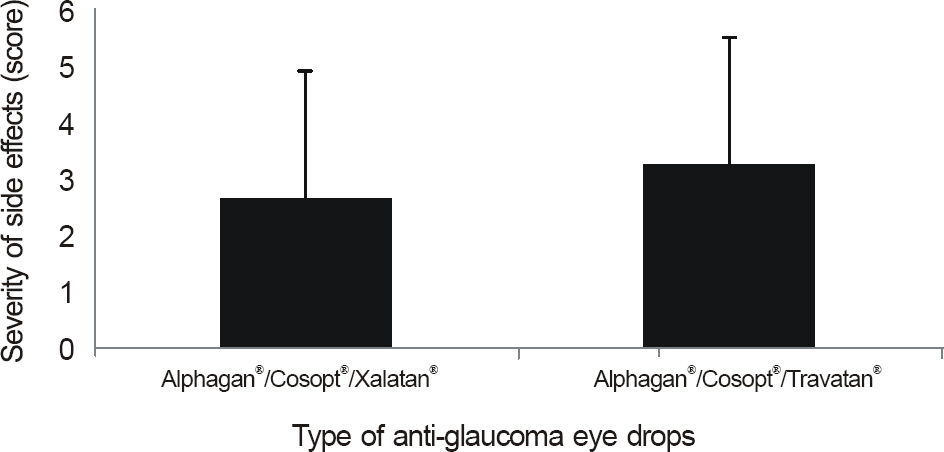
The side effects after the use of anti-glaucoma eye drops were more severe and frequent in the patient groups using more than 1 eye drop (p < 0.01) and in the patients with longer treatment period (p < 0.01). Among patients using 1 eye drops, side effects developed frequently in patients using Ocucarpine(R).
CONCLUSIONS
In patients with glaucoma, using more than 1 anti-glaucoma eye drop or having a longer treatment period resulted in more side effects. Similaryly, patients using only 1 eye drop of Ocu-carpine(R) induced more side effects.
MeSH Terms
Figure
Cited by 1 articles
-
Glaucoma Schlemm's canal stent insertion: a systematic review
Sunyoung Jo
J Korean Med Assoc. 2016;59(8):637-643. doi: 10.5124/jkma.2016.59.8.637.
Reference
-
References
1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80:389–93.
Article2. Goldmann H, Schmidt T. Applanation tonometry. Ophthalmologica. 1957; 134:221–42.3. Shazly TA, Latina MA. Comparison of intraocular pressure-low-ering effect of every night versus every other night dosing of bima-toprost 0.03%. J Ocul Pharmacol Ther. 2011; 27:369–71.
Article4. Collaborative Normal-Tension Glaucoma Study Group. The effec-tiveness of intraocular pressure reduction in the treatment of nor-mal-tension glaucoma. Am J Ophthalmol. 1998; 126:498–505.5. The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000; 130:429–40.6. Katsanos A, Dastiridou AI, Fanariotis M, et al. Bimatoprost and bi-matoprost/timolol fixed combination in patients with open-angle glaucoma and ocular hypertension. J Ocul Pharmacol Ther. 2011; 27:67–71.
Article7. Schuman JS. Antiglaucoma medications: a review of safety and tolerability issues related to their use. Clin Ther. 2000; 22:167–208.
Article8. Pfister RR, Burstein N. The effects of ophthalmic drugs, vehicles, and preservatives on corneal epithelium: a scanning electron mi-croscope study. Invest Ophthalmol. 1976; 15:246–59.9. Burstein NL. Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine digluconate in cat and rabbit corneas. Invest Ophthalmol Vis Sci. 1980; 19:308–13.10. Tripathi BJ, Tripathi RC, Kolli SP. Cytotoxicity of ophthalmic preservatives on human corneal epithelium. Lens Eye Toxic Res. 1992; 9:361–75.11. Alm A, Stjernschantz J. Effects on intraocular pressure and side ef-fects of 0.005% latanoprost applied once daily, evening or morning. A comparison with timolol. Scandinavian Latanoprost Study Group. Ophthalmology. 1995; 102:1743–52.12. Burtein NL. Corneal cytotoxicity of topically applied drugs, ve-hicles and preservatives. Surv Ophthalmol. 1980; 25:15–30.13. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symp-toms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002; 86:418–23.
Article14. Lavin MJ, Wormald RP, Migdal CS, Hitchings RA. The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol. 1990; 108:1543–8.
Article15. Richter CU, Shingleton BJ, Bellows AR, et al. The development of encapsulated filtering blebs. Ophthalmology. 1988; 95:1163–8.16. Broadway D, Grierson I, Hitchings R. Adverse effects of topical antiglaucomatous medications on the conjunctiva. Br J Ophthalmol. 1993; 77:590–6.
Article17. Broadway DC, Grierson I, O`Brien C, Hitchings RA. Adverse ef-fects of topical antiglaucoma medication. II. The outcome of filtra-tion surgery. Arch Ophthalmol. 1994; 112:1446–54.
Article18. Ashburn FS Jr, Goldberg I, Kass MA. Compliance with ocular therapy. Surv Ophthalmol. 1980; 24:237–48.
Article19. Ahn DH, Lee YG, Hong YJ. Factors affecting compliance with prescribed eyedrops for glaucoma. Korean J Ophthalmol Soc. 1998; 39:2145–51.20. Beckers HJ, Schouten JS, Webers CA, et al. Side effects of com-monly used glaucoma medications: comparison of tolerability, chance of discontinuation, and patient satisfaction. Graefes Arch Clin Exp Ophthalmol. 2008; 246:1485–90.
Article21. Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma. 2012; 21:234–40.
Article22. Robin A, Grover DS. Compliance and adherence in glaucoma management. Indian J Ophthalmol. 2011; 59 Suppl:S93–6.
Article23. Granström PA. Glaucoma patient not compliant with their drug therapy: clinical and behavioural aspects. Br J Ophthalmol. 1982; 66:464–70.24. Berdy GJ, Abelson MB, Smith LM, George MA. Preservative-free artificial tear preparations. Assessment of corneal epithelial toxic effects. Arch Ophthalmol. 1992; 110:528–32.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Importance of Evaluation of Corneal Endothelial Cell Count in Glaucoma Patients
- A Clinical Study of Topical 2% Dorzolamide(Trusopt) as Adjunctive Therapy
- The Effects of Anti-glaucoma Eyedrops on Corneal Hysteresis in Patients with Open-angle Glaucoma and Glaucoma-suspect
- Short-term Efficacy and Safety of Omidenepag Isopropyl 0.002% w/v Therapy for Patients with Primary Open-angle Glaucoma and Ocular Hypertension
- Effects of anti-glaucoma drugs on resistive index of the medial long posterior ciliary artery using color Doppler imaging in Beagle dogs