J Korean Ophthalmol Soc.
2013 Apr;54(4):639-644. 10.3341/jkos.2013.54.4.639.
Effect of Nitric Oxide on Adhesion and Migration of Trabecular Meshwork Cells
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2216946
- DOI: http://doi.org/10.3341/jkos.2013.54.4.639
Abstract
- PURPOSE
To investigate the effect of nitric oxide (NO) on the adhesion and migration of cultured human trabecular meshwork cells (HTMC).
METHODS
For adhesion assay, primarily cultured HTMC were attached to culture dishes for 1 hr, cells were rinsed, and the remaining adherent cells were assessed with MTT assay. Degree of cellular migration was assessed under normal and stressed conditions using microchemoattraction chambers. Effect of NO on the adhesion and migration was assessed with or without co-exposure of S-nitroso-N-acetylpenicillamine (SNAP).
RESULTS
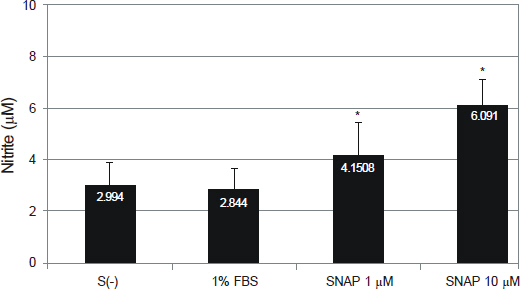
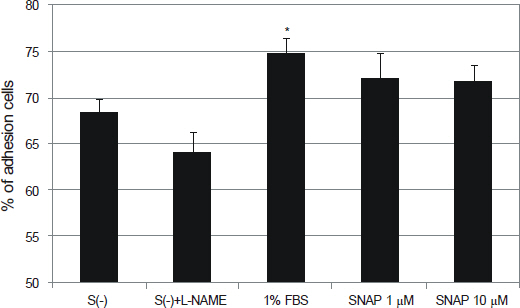
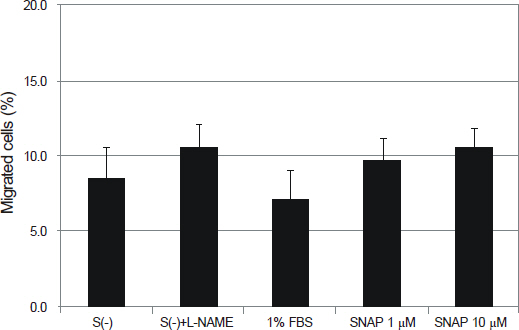
NO did not affect the degree of adhesion or migration of HTMC (p > 0.05). The degree of adhesion increased although the degree of migration decreased with 1% serum (p < 0.05). Degrees of migrations decreased after mechanical stress (p < 0.05).
CONCLUSIONS
NO may not affect the adhesion or migration of HTMC.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.2. Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994; 63:175–95.
Article3. Park GS, Kwon NS, Kim YM, Kim JC. The role of nitric oxide in ocular surface diseases. Korean J Ophthalmol. 2001; 15:59–66.
Article4. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.5. Alvarado JA, Alvarado RG, Yeh RF, et al. A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm's canal endothelial cells. Br J Ophthalmol. 2005; 89:1500–5.
Article6. Hattenbach LO, Allers A, Klais C, et al. L-Arginine-nitric oxide pathway-related metabolites in the aqueous humor of diabetic patients. Invest Ophthalmol Vis Sci. 2000; 41:213–7.7. Matsuo T. Basic nitric oxide production is enhanced by hydraulic pressure in cultured human trabecular cells. Br J Ophthalmol. 2000; 84:631–5.8. Haefliger IO, Dettmann E, Liu R, et al. Potential role of nitric oxide and endothelin in the pathogenesis of glaucoma. Surv Ophthalmol. 1999; 43:S51–8.
Article9. Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.10. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp Eye Res. 1994; 58:99–105.
Article11. Wana RF, Podos SM. Effect of the topical application of nitroglycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995; 60:337–9.12. Gipson IK, Anderson RA. Actin filaments in cells of human trabecular meshwork and Schlemm's canal. Invest Ophthalmol Vis Sci. 1979; 18:547–61.13. Noiri E, Hu Y, Bahou WF, et al. Permissive role of nitric oxide in endothelin-induced migration of endothelial cells. J Biol Chem. 1997; 272:1747–52.
Article14. Goligorsky MS, Abedi H, Noiri E, et al. Nitric oxide modulation of focal adhesions in endothelial cells. Am J Physiol. 1999; 276:C1271–81.15. Kiviluoto T, Watanabe S, Hirose M, et al. Nitric oxide donors retard wound healing in cultured rabbit gastric epithelial cell monolayers. Am J Physiol Gastrointest Liver Physiol. 2001; 281:G1151–7.
Article16. Kolpakov V, Gordon D, Kulik TJ. Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ Res. 1995; 76:305–9.
Article17. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–79.
Article18. Nakamura Y, Sagara T, Seki K, et al. Permissive effect of fibronectin on collagen gel contraction mediated by bovine trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003; 44:4331–6.
Article19. Ignarro LJ, Lippton H, Edwards JC, et al. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprus-side, and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981; 218:739–49.20. Schröder H, Noack E, Müller R. Evidence for a correlation between nitric oxide formation by cleavage of organic nitrates and activation of guanylate cyclase. J Mol Cell Cardiol. 1985; 17:931–4.21. Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989; 83:1774–7.
Article22. Green LC, Wagner DA, Glogoski J, et al. Analysis of nitrate, nitrite and [15N]nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.23. Okouchi M, Okayama, N, Shimizu , et al. High insulin exacerbates neutrophil-endothelial cell adhesion through endothelial surface expression of intercellular adhesion molecule-1 via activation of protein kinase C and mitogen-activated protein kinase. Diabetologia. 2002; 45:556–9.
Article24. Hirata F, Yoshida M, Ogura Y. High glucose exacerbates neutrophil adhesion to human retinal endothelial cells. Exp Eye Res. 2006; 82:179–82.
Article25. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article26. Hogg P, Calthorpe M, Batterbury M, Grierson I. Aqueous humor stimulates the migration of human trabecular meshwork cells in vitro. Invest Ophthalmol Vis Sci. 2000; 41:1091–8.27. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–9.28. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–78.29. Schaffer M, Tantry U, Gross SS, et al. Nitric oxide regulates wound healing. J Surg Res. 1996; 63:237–40.30. Schwentker A, Vodovotz Y, Weller R, Billar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002; 7:1–10.
Article31. Noiri E, Hu Y, Bahou WF, et al. Permissive role of nitric oxide in endothelin-induced migration of endothelial cells. J Biol Chem. 1997; 272:1747–52.
Article32. Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996; 7:1209–24.
Article33. Ilić D, Furuta Y, Kanazawa S, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995; 377:539–44.
Article34. McCuskey RS, Urbaschek R, Urbaschek B. The microcirculation during endotoxemia. Cardiovasc Res. 1996; 32:752–63.
Article35. Rohen JW, van der, Zypen E. The phagocytic activity of the trabecularmeshwork endothelium. An electron-microscopic study of the vervet (Cercopithecus aethiops). Albrecht Von Graefes Arch Clin Exp Ophthalmol. 1968; 175:143–60.36. Shabo AL, Maxwell DS. Observations on the fate of blood in the anterior chamber. A light and electron microscopic study of the monkey trabecular meshwork. Am J Ophthalmol. 1972; 73:25–36.
Article37. Grierson I, Lee WR. Erythrocyte phagocytosis in the human trabecular meshwork. Br J Ophthalmol. 1973; 57:400–15.
Article38. Grierson I, Chisholm IA. Clearance of debris from the iris through the drainage angle of the rabbit's eye. Br J Ophthalmol. 1978; 62:694–704.
Article39. Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond). 1987; 1:204–10.
Article40. Grierson I, Hogg P. The proliferative and migratory activities of trabecular meshwork cells. Prog Retin Eye Res. 1995; 15:33–67.
Article41. Hogg P, Calthorpe CM, Ward S, Grierson I. Migration of cultured bovine trabecular meshwork cells to aqueous humor and constituents. Invest Ophthalmol Vis Sci. 1995; 36:2449–60.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Mitomycin C on the Proliferation and Nitric Oxide Production in the Cultured Trabecular Meshwork Cells
- Effect of beta-adrenergics on the Survival and Production of Nitric Oxide in the Cultured Trabecular Meshwork Cells
- Effect of Erythropoietin on the Production of Nitric Oxide in Trabecular Meshwork Cells
- Effects of Triamcinolone Acetonide in Cultured Trabecular Meshwork Cells
- Effect of Nitric Oxide on the Expression of Matrix Metalloproteinase and Its Association with Migration of Cultured Trabecular Meshwork Cells