J Korean Ophthalmol Soc.
2012 Sep;53(9):1352-1356. 10.3341/jkos.2012.53.9.1352.
Intravitreal Bevacizumab Injection for Choroidal Neovascularization Secondary to Laser Photocoagulation for Central Serous Chorioretinopathy
- Affiliations
-
- 1Department of Ophthalmology, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea. eyedr@dsmc.or.kr
- 2Department of Ophthalmology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- KMID: 2216092
- DOI: http://doi.org/10.3341/jkos.2012.53.9.1352
Abstract
- PURPOSE
To report a case of intravitreal bevacizumab injection for choroidal neovascularization following direct laser photocoagulation for central serous chorioretinopathy.
CASE SUMMARY
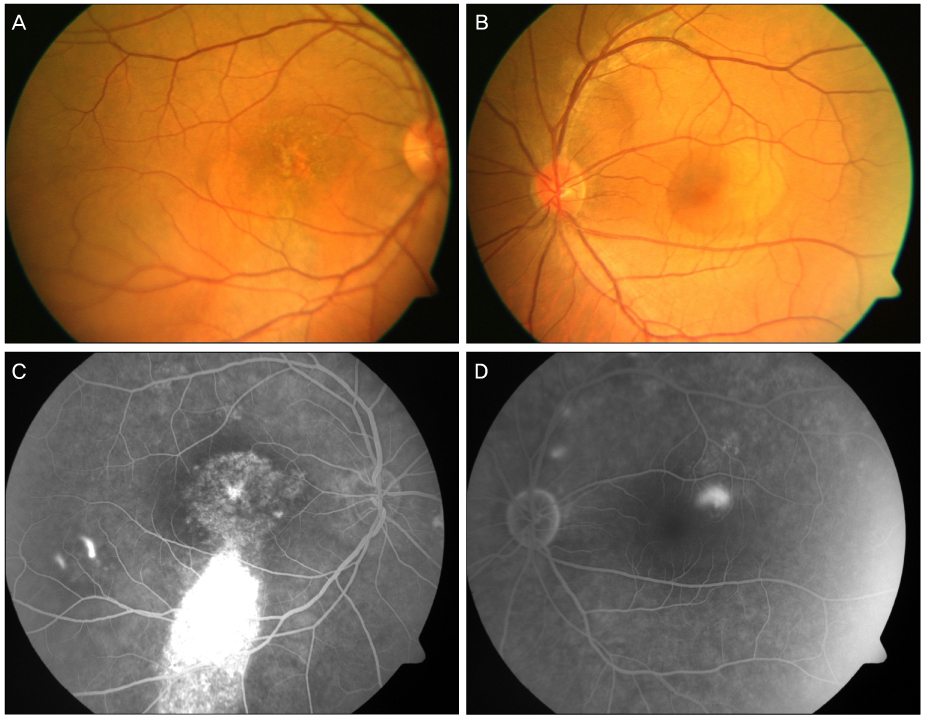
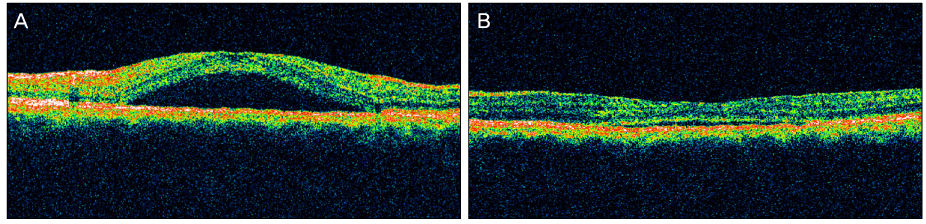
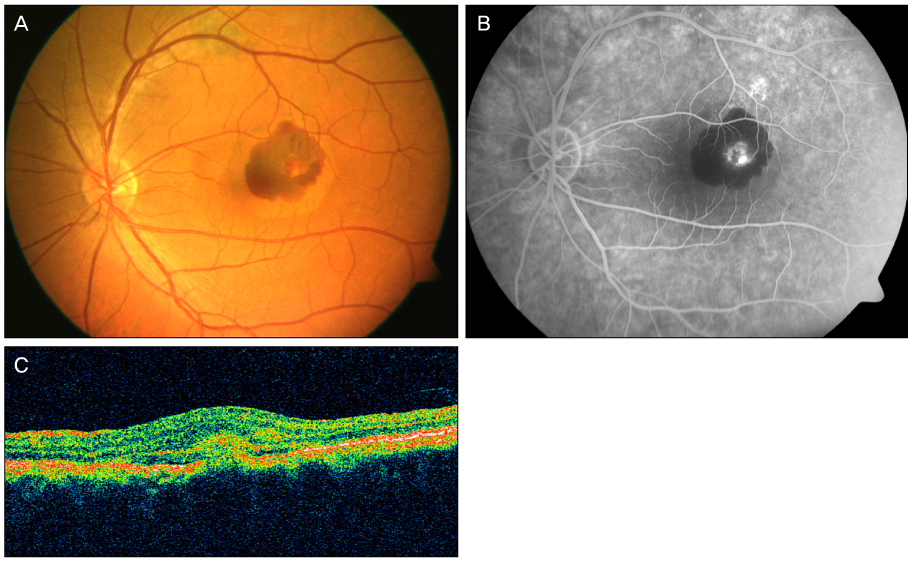
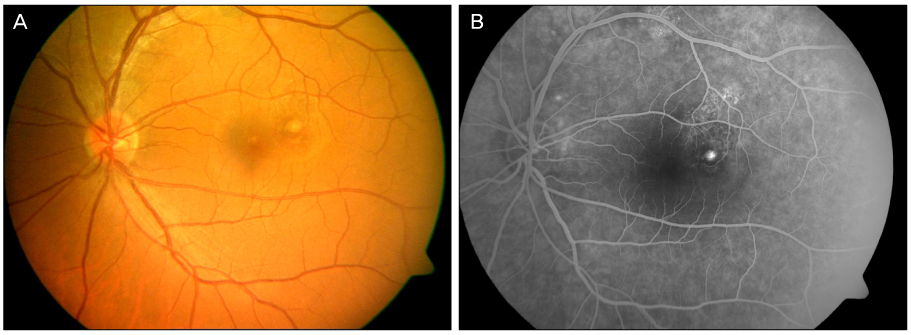
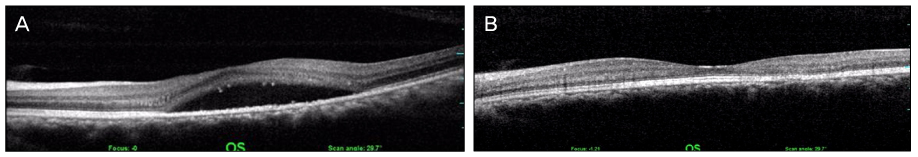
A 44-year-old male patient with an 8-month history of metamorphopsia in his left eye visited our clinic and was diagnosed with central serous chorioretinopathy after performing refraction, fundus examination, fluorescein angiography (FAG) and optical coherence tomography (OCT). After 1 month, laser photocoagulation of the leaking point observed on the FAG was performed. After 8 weeks following laser photocoagulation, visual acuity was reduced to 0.4, subretinal hemorrhage accompanied by choroidal neovascularization was observed on FAG and OCT, and an intravitreal bevacizumab injection was administered. After 4 weeks following the injection, macular edema and subretinal hemorrhage decreased, visual acuity increased to 1.0 and was maintained properly. However, after 2 years, the central serous chorioretinopathy recurred and after 3 months, healed spontaneously.
CONCLUSIONS
Intravitreal bevacizumab injection is a safe and effective treatment for secondary choroidal neovascularization occurring after direct laser photocoagulation for central serous chorioretinopathy. In addition, a single treatment can maintain the patient's status with no recurrence of choroidal neovascularization over a long-term period.
MeSH Terms
Figure
Reference
-
1. Clais CM, Ober DM, Ciardella AP, Yannuzzi LA. Ryan SJ, editor. Central serous chorioretinopathy. Retina. 2006. Vol. 2:4th ed. Philadelphia: Elsevier-Mosby;1136–1161.2. Burumcek E, Mudun A, Karacorlu S, Arslan MO. Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology. 1997. 104:616–622.3. Gass JD. Photocoagulation treatment of idiopathic central serous choroidopathy. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977. 83(3 Pt 1):456–467.4. Matsunaga H, Nangoh K, Uyama M, et al. [Occurrence of choroidal neovascularization following photocoagulation treatment for central serous retinopathy]. Nihon Ganka Gakkai Zasshi. 1995. 99:460–468.5. Ha TW, Ham DI, Kang SW. Management of choroidal neovascularization following laser photocoagulation for central serous chorioretinopathy. Korean J Ophthalmol. 2002. 16:88–92.6. Chan WM, Li KK, Liu DT, et al. Photodynamic therapy with verteporfin in laser-induced choroidal neovascularization. Am J Ophthalmol. 2003. 136:565–567.7. Pikkel J, Rumelt S. Intravitreal bevacizumab for choroidal neovascularization secondary to laser photocoagulation for central serous chorioretinopathy. Eur J Ophthalmol. 2012. 22:488–491.8. Cakir M, Cekiç O, Yilmaz OF. Photodynamic therapy for iatrogenic CNV due to laser photocoagulation in central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging. 2009. 40:405–408.9. Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984. 68:815–820.10. Chung SE, Kang JH, Kang SW. Chronic central serous chorioretinopathy: photodynamic therapy. J Korean Ophthalmol Soc. 2007. 48:279–284.11. Oh J, Kwon OW, Kim MH, et al. Photodynamic therapy for chronic central serous chorioretinopathy: multicenter study of 65 cases. J Korean Ophthalmol Soc. 2009. 50:390–398.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laser-Induced Choroidal Neovascularization in Central Serous Chorioretinopathy: 4 Cases
- Comparison between Focal Laser Photocoagulation and Intravitreal Bevacizumab Injection for Treating Chronic Central Serous Chorioretinopathy
- Comparison of Intravitreal Bevacizumab and Aflibercept Injections for Central Serous Chorioretinopathy
- Management of choroidal neovascularization following laser photocoagulation for central serous chorioretinopathy
- The Short-term Effect of Intravitreal Bevacizumab for Treatment of Central Serous Chorioretinopathy