J Korean Ophthalmol Soc.
2016 May;57(5):786-793. 10.3341/jkos.2016.57.5.786.
The Repeatability of Retinal Layer Thickness Measurements with Spectral-Domain Optical Coherence Tomography in Normal Eyes
- Affiliations
-
- 1Department of Ophthalmology, Kyung Hee University Hospital, Kyung Hee University School of Medicine, Seoul, Korea. syyu@khu.ac.kr
- KMID: 2212722
- DOI: http://doi.org/10.3341/jkos.2016.57.5.786
Abstract
- PURPOSE
To evaluate the repeatability of retinal layer thickness measurements in normal eyes imaged using spectral domain optical coherence tomography (SD-OCT).
METHODS
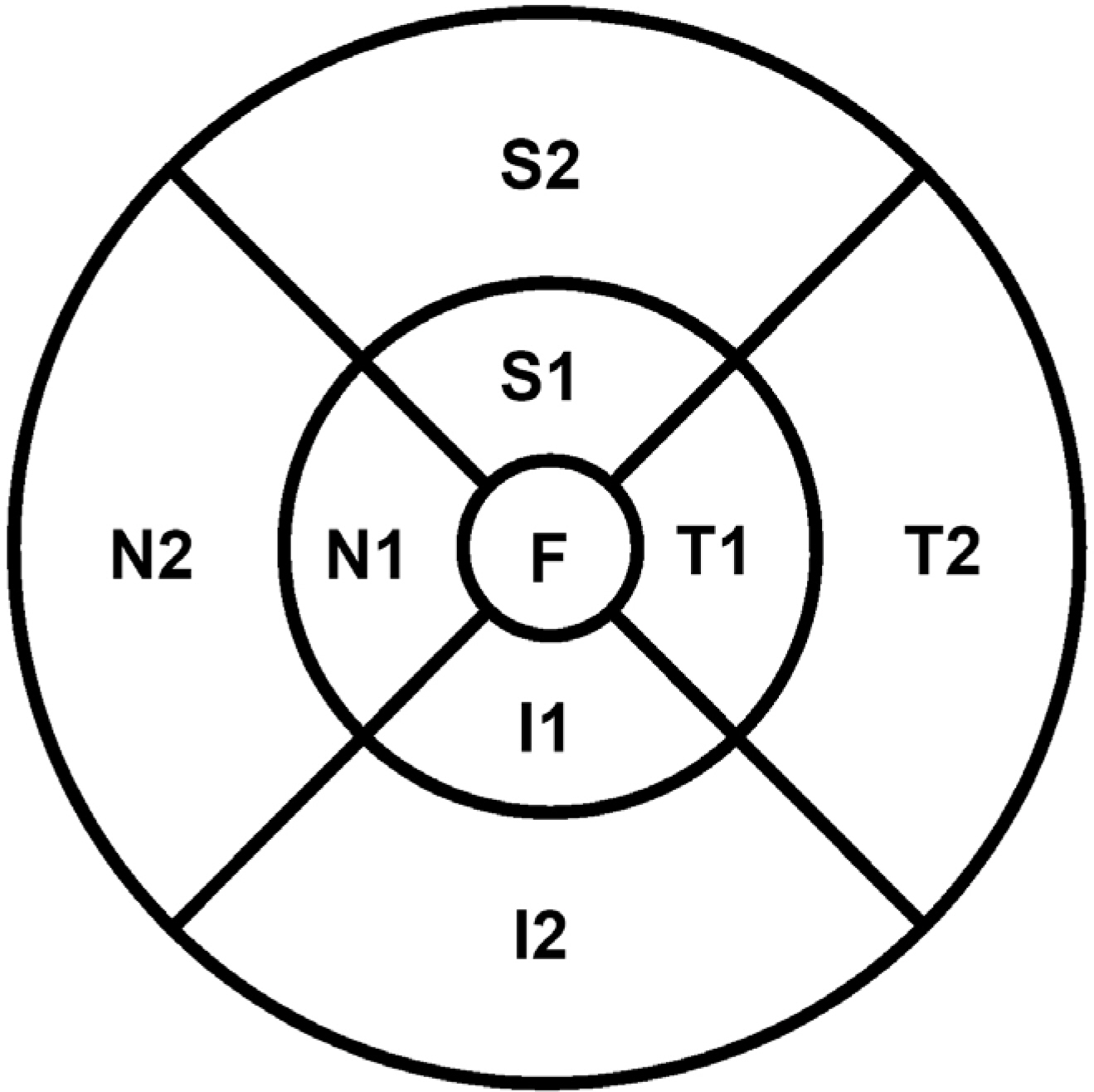
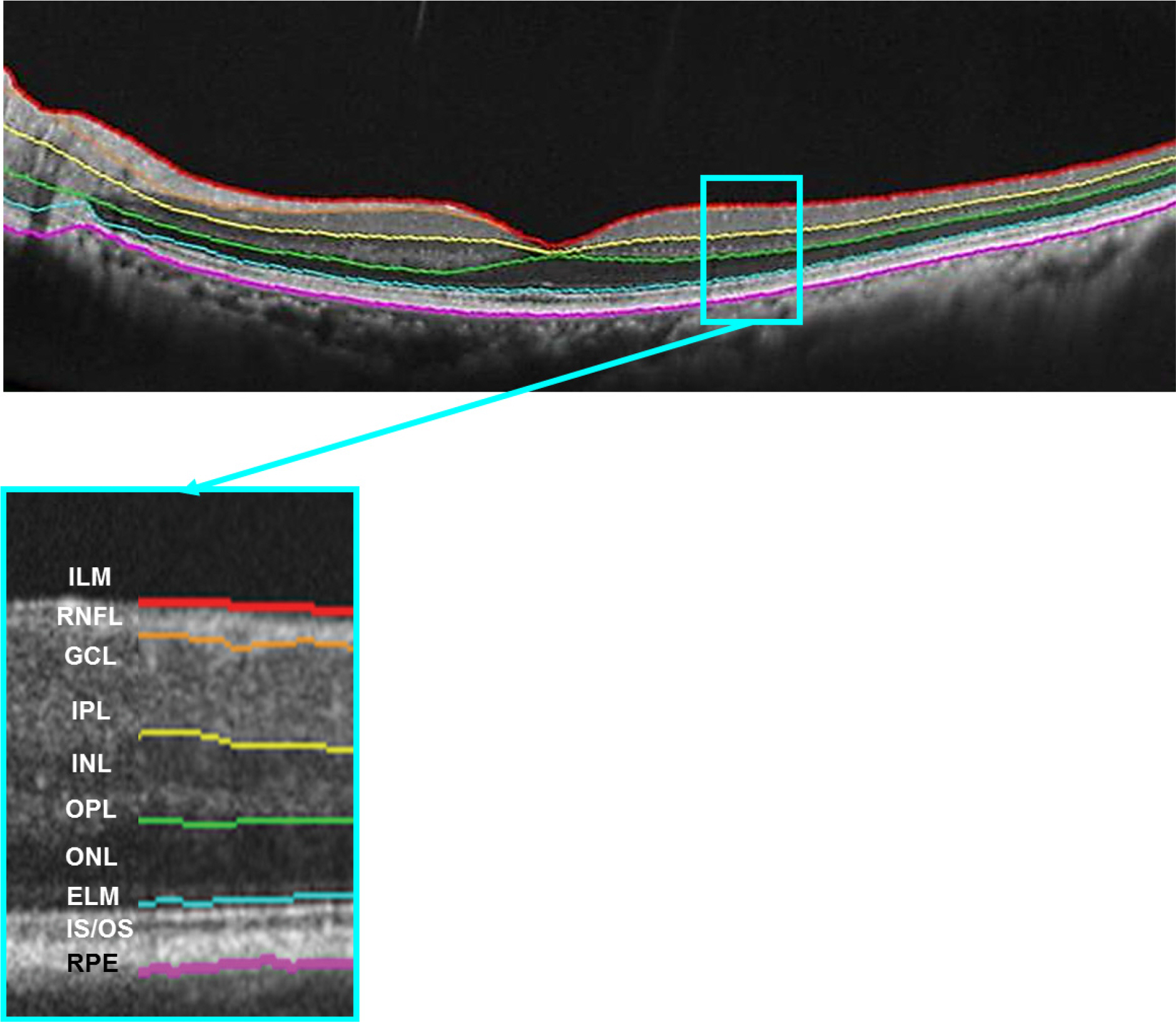
Sixty-eight eyes of 34 healthy subjects were enrolled in this study. Imaging was performed 4 times using 9 × 9 mm macular scans with SD-OCT (RS 3000 Advance HD OCT, NIDEK, Gamagori, Japan) at the same visit by an experienced examiner. After automatic retinal segmentation (layering) in 5 layers, the thickness of each layer was calculated. Macular thickness of 9 Early Treatment of Diabetic Retinopathy Study (ETDRS)-like regions was obtained. Repeatability for each of the 9 subfield areas was calculated by their repeatability coefficients and intraclass correlation coefficients (ICCs).
RESULTS
There was no significant difference in average retinal thickness and each retinal layer thickness between all measurements acquired by the experienced examiner. The ICCs of retinal layer thickness ranged from 0.826 to 0.847 for the ganglion cell layer + inner plexiform layer, inner nuclear layer + outer plexiform layer and outer nuclear layer + external limiting membrane in the fovea. The ICCs were greater than 0.909 for the other intra-retinal layers in all 9 ETDRS subfield thickness between all measurement pairs.
CONCLUSIONS
Excellent repeatability was observed for SD-OCT retinal segmented layer thickness measurements in healthy subjects.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Leung CK. Cheung CY. Weinreb RN, et al. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2008; 49:4893–7.
Article2. Menke MN. Dabov S. Knecht P. Sturm V. Reproducibility of retinal thickness measurements in healthy subjects using spectralis optical coherence tomography. Am J Ophthalmol. 2009; 147:467–72.
Article3. Han IC. Jaffe GJ. Comparison of spectral- and time-domain optical coherence tomography for retinal thickness measurements in healthy and diseased eyes. Am J Ophthalmol. 2009; 147:847–58. 858.e1.
Article4. Vizzeri G. Weinreb RN. Gonzalez-Garcia AO, et al. Agreement between spectral-domain and time-domain OCT for measuring RNFL thickness. Br J Ophthalmol. 2009; 93:775–81.
Article5. Hess DB. Asrani SG. Bhide MG, et al. Macular and retinal nerve fiber layer analysis of normal and glaucomatous eyes in children using optical coherence tomography. Am J Ophthalmol. 2005; 139:509–17.
Article6. Leung CK. Mohamed S. Leung KS, et al. Retinal nerve fiber layer measurements in myopia: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2006; 47:5171–6.
Article7. Kotowski J. Folio LS. Wollstein G, et al. Glaucoma discrimination of segmented cirrus spectral domain optical coherence tomography (SD-OCT) macular scans. Br J Ophthalmol. 2012; 96:1420–5.
Article8. Matsumoto H. Sato T. Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am J Ophthalmol. 2009; 148:105–10. e1.
Article9. Acton JH. Smith RT. Hood DC. Greenstein VC. Relationship between retinal layer thickness and the visual field in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012; 53:7618–24.
Article10. Huang J. Liu X. Wu Z, et al. Macular thickness measurements in normal eyes with time-domain and Fourier-domain optical coherence tomography. Retina. 2009; 29:980–7.
Article11. Mansouri K. Medeiros FA. Tatham AJ, et al. Evaluation of retinal and choroidal thickness by swept-source optical coherence tomography: repeatability and assessment of artifacts. Am J Ophthalmol. 2014; 157:1022–32.
Article12. Muscat S. Parks S. Kemp E. Keating D. Repeatability and reproducibility of macular thickness measurements with the Humphrey OCT system. Invest Ophthalmol Vis Sci. 2002; 43:490–5.13. Oh SB. Cho WB. Moon JW. Kim HC. Repeatability and agreement of macular thickness measurement using time domain OCT and spectral domain OCT in normal subjects. J Korean Ophthalmol Soc. 2009; 50:710–16.
Article14. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985; 103:1796–806.15. Pierro L. Gagliardi M. Iuliano L, et al. Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Invest Ophthalmol Vis Sci. 2012; 53:5912–20.
Article16. Kiernan DF. Zelkha R. Hariprasad SM, et al. En face spectral-domain optical coherence tomography outer retinal analysis and relation to visual acuity. Retina. 2012; 32:1077–86.
Article17. Mohammad F. Wanek J. Zelkha R, et al. A method for En Face OCT Imaging of subretinal fluid in age-related macular degeneration. J Ophthalmol. 2014; 2014:720243.
Article18. Geitzenauer W. Hitzenberger CK. Schmidt-Erfurth UM. Retinal optical coherence tomography: past, present and future perspectives. Br J Ophthalmol. 2011; 95:171–7.
Article19. Watanabe A. Arimoto S. Nishi O. Correlation between metamorphopsia and epiretinal membrane optical coherence tomography findings. Ophthalmology. 2009; 116:1788–93.
Article20. Koo HC. Rhim WI. Lee EK. Morphologic and functional association of retinal layers beneath the epiretinal membrane with spectral-domain optical coherence tomography in eyes without photoreceptor abnormality. Graefes Arch Clin Exp Ophthalmol. 2012; 250:491–8.
Article21. Moon SW. Kim ES. Kim YG, et al. The comparison of macular thickness measurements and repeatabilities between time domain and spectral domain OCT. J Korean Ophthalmol Soc. 2009; 50:1050–9.
Article22. Carpineto P. Aharrh-Gnama A. Ciciarelli V, et al. Reproducibility and repeatability of ganglion cell-inner plexiform layer thickness measurements in healthy subjects. Ophthalmologica. 2014; 232:163–9.
Article23. Ng DS. Gupta P. Tham YC, et al. Repeatability of perimacular ganglion cell complex analysis with spectral-domain optical coherence tomography. J Ophthalmol. 2015; 2015:605940.
Article24. Müller R. Büttner P. A critical discussion of intraclass correlation coefficients. Stat Med. 1994; 13:2465–76.
Article25. Kang NH. Kim HJ. Lee JH. The measurements of macular thickness and volume with SD-OCT in normal eyes. J Korean Ophthalmol Soc. 2011; 52:1182–8.
Article26. Polito A. Shah SM. Haller JA, et al. Comparison between retinal thickness analyzer and optical coherence tomography for assessment of foveal thickness in eyes with macular disease. Am J Ophthalmol. 2002; 134:240–51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repeatability of Spectral Domain OCT (3D-OCT 1000) in Normal Subjects and Various Macular Diseases
- Thickness of the Macula, Retinal Nerve Fiber Layer, and Ganglion Cell-inner Plexiform Layer in the Macular Hole: The Repeatability Study of Spectral-domain Optical Coherence Tomography
- Spectral-Domain Optical Coherence Tomography Findings in Acute Central Retinal Artery Occlusion
- Retinal Nerve Fiber Layer Thickness Measured by Spectral Domain Optical Coherence Tomography in Healthy Koreans
- Difference of GCIPL Thickness of Diabetes and Normal Eyes in Spectral Domain OCT