Korean J Ophthalmol.
2018 Dec;32(6):506-516. 10.3341/kjo.2018.0030.
Thickness of the Macula, Retinal Nerve Fiber Layer, and Ganglion Cell-inner Plexiform Layer in the Macular Hole: The Repeatability Study of Spectral-domain Optical Coherence Tomography
- Affiliations
-
- 1Department of Ophthalmology, Chungnam National University College of Medicine, Daejeon, Korea. kimjy@cnu.ac.kr
- KMID: 2427966
- DOI: http://doi.org/10.3341/kjo.2018.0030
Abstract
- PURPOSE
We measured the thicknesses of the ganglion cell and inner plexiform layer (GCIPL), the macula, and the retinal nerve fiber layer (RNFL) using spectral-domain optical coherence tomography in patients with idiopathic macula holes to analyze the repeatability of these measurements and compare them with those of the fellow eye.
METHODS
We evaluated 85 patients who visited our retinal clinic. The patients were divided into two groups according to their macular hole size: group A had a size of <400 µm, while group B had a size of ≥400 µm. Repeatability was determined by comparing the thicknesses of the GCIPL, macula, and RNFL with those of the normal fellow eye.
RESULTS
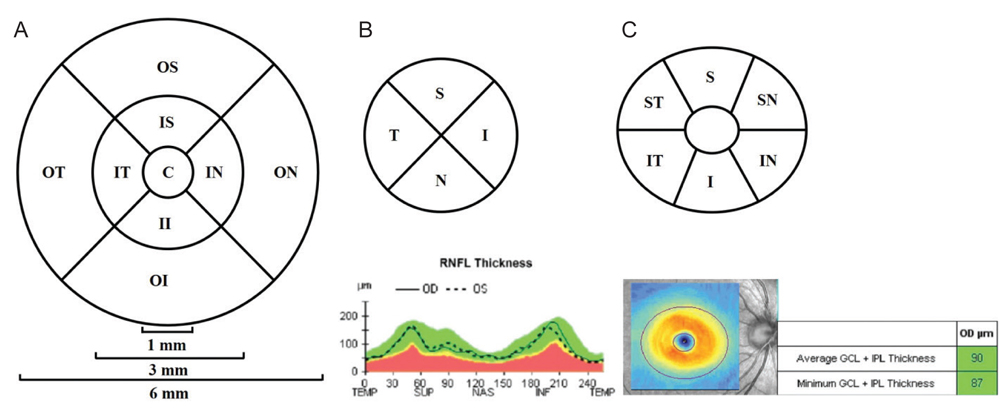
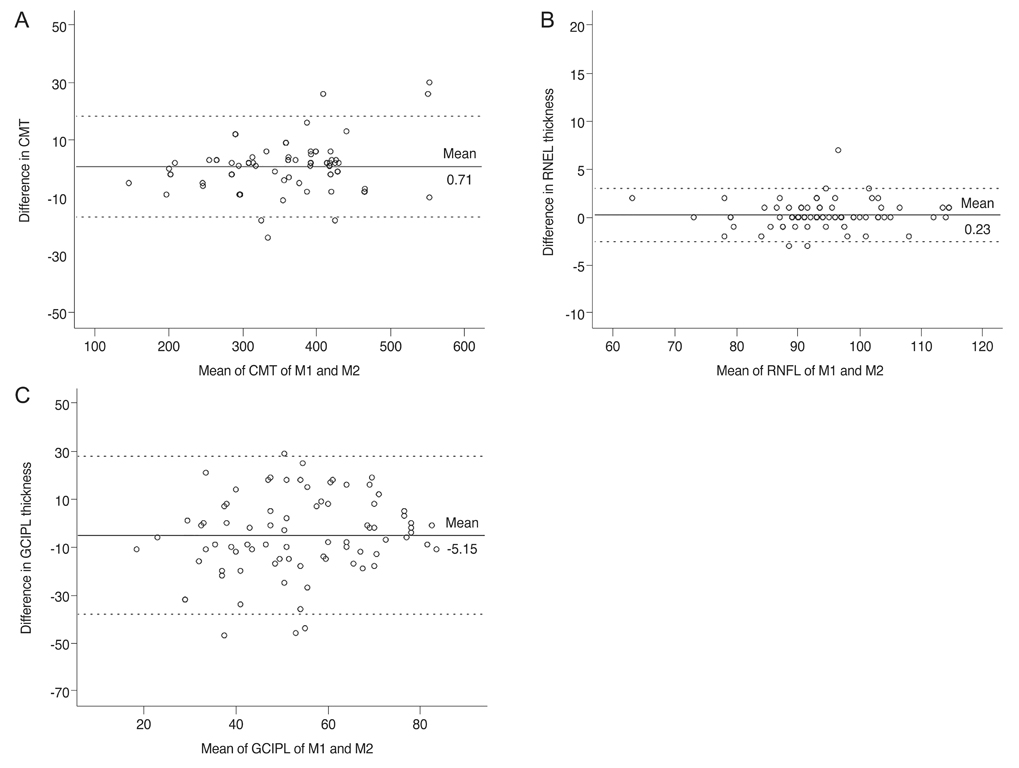
The average central macular thickness in patients with macular holes was significantly thicker than that in the normal fellow eye (343.8 ± 78.6 vs. 252.6 ± 62.3 µm, p < 0.001). The average thickness of the GCIPL in patients with macular holes was significantly thinner than that in the normal fellow eye (56.1 ± 23.4 vs. 77.1 ± 12.8 µm, p < 0.001). There was no significant difference in the average RNFL thickness between eyes with macular holes and fellow eyes (92.4 ± 10.0 vs. 95.5 ± 10.7 µm, p = 0.070). There were also no significant differences in the thicknesses of the GCIPL and RNFL among the two groups (p = 0.786 and p = 0.516). The intraclass correlation coefficients for the macula and RNFL were 0.994 and 0.974, respectively, in patients with macular holes, while that for the GCIPL was 0.700.
CONCLUSIONS
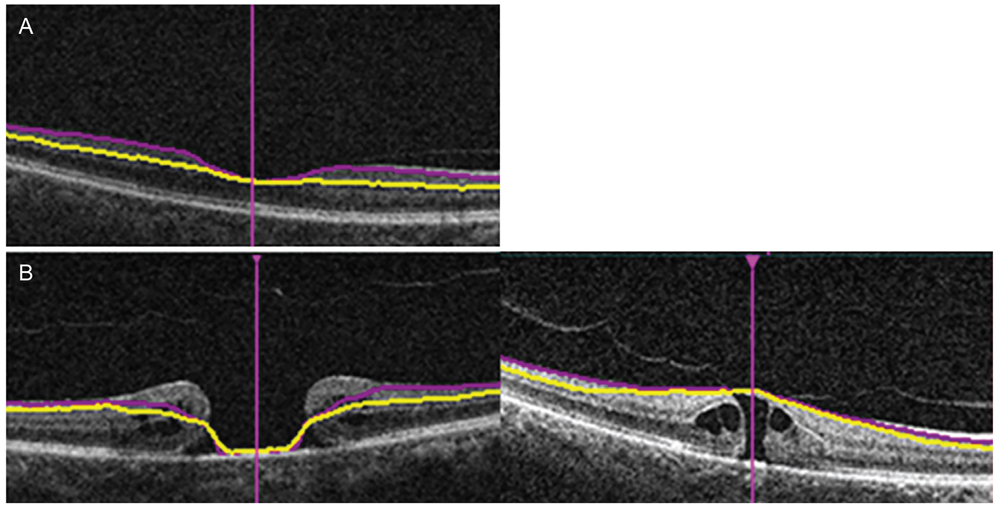
Macular contour change with macular hole results in low repeatability and a tendency of thinner measurement regarding GCIPL thickness determined via spectral-domain optical coherence tomography. The impact of changes in the macular shape caused by macular holes should be taken into consideration when measuring the GCIPL thickness in patients with various eye diseases such as glaucoma and in those with neuro-ophthalmic disorders.
MeSH Terms
Figure
Reference
-
1. la Cour M, Friis J. Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmol Scand. 2002; 80:579–587.
Article2. Knapp H. About isolated ruptures of the choroid as a result of trauma on the eyeball [Uber isolierte Zerreissungen der Aderhaut in Folge von Traumen auf dem Augapfel]. Arch Augenheilkd. 1869; 1:6–29.3. Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995; 119:752–759.
Article4. Gaudric A, Haouchine B, Massin P, et al. Macular hole formation: new data provided by optical coherence tomography. Arch Ophthalmol. 1999; 117:744–751.5. Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology. 1995; 102:748–756.
Article6. de Sisternes L, Hu J, Rubin DL, Leng T. Visual prognosis of eyes recovering from macular hole surgery through automated quantitative analysis of spectral-domain optical coherence tomography (SD-OCT) scans. Invest Ophthalmol Vis Sci. 2015; 56:4631–4643.
Article7. Xu D, Yuan A, Kaiser PK, et al. A novel segmentation algorithm for volumetric analysis of macular hole boundaries identified with optical coherence tomography. Invest Ophthalmol Vis Sci. 2013; 54:163–169.
Article8. Krebs I, Hagen S, Brannath W, et al. Repeatability and reproducibility of retinal thickness measurements by optical coherence tomography in age-related macular degeneration. Ophthalmology. 2010; 117:1577–1584.
Article9. Mwanza JC, Budenz DL, Godfrey DG, et al. Diagnostic performance of optical coherence tomography ganglion cell: inner plexiform layer thickness measurements in early glaucoma. Ophthalmology. 2014; 121:849–854.10. Mwanza JC, Oakley JD, Budenz DL, et al. Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2011; 52:8323–8329.
Article11. Moon H, Yoon JY, Lim HT, Sung KR. Ganglion cell and inner plexiform layer thickness determined by spectral domain optical coherence tomography in patients with brain lesions. Br J Ophthalmol. 2015; 99:329–335.
Article12. Francoz M, Fenolland JR, Giraud JM, et al. Reproducibility of macular ganglion cell-inner plexiform layer thickness measurement with cirrus HD-OCT in normal, hypertensive and glaucomatous eyes. Br J Ophthalmol. 2014; 98:322–328.
Article13. Lee HJ, Kim MS, Jo YJ, Kim JY. Thickness of the macula, retinal nerve fiber layer, and ganglion cell layer in the epiretinal membrane: the repeatability study of optical coherence tomography. Invest Ophthalmol Vis Sci. 2015; 56:4554–4559.
Article14. Hirasawa K, Shoji N, Yoshii Y, Haraguchi S. Determination of axial length requiring adjustment of measured circumpapillary retinal nerve fiber layer thickness for ocular magnification. PLoS One. 2014; 9:e107553.
Article15. Folio LS, Wollstein G, Ishikawa H, et al. Variation in optical coherence tomography signal quality as an indicator of retinal nerve fibre layer segmentation error. Br J Ophthalmol. 2012; 96:514–518.
Article16. Cantrill HL. The diabetic retinopathy study and the early treatment diabetic retinopathy study. Int Ophthalmol Clin. 1984; 24:13–29.
Article17. Muller R, Buttner P. A critical discussion of intraclass correlation coefficients. Stat Med. 1994; 13:2465–2476.18. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–310.
Article19. Barak Y, Ihnen MA, Schaal S. Spectral domain optical coherence tomography in the diagnosis and management of vitreoretinal interface pathologies. J Ophthalmol. 2012; 2012:876472.
Article20. Takahashi H, Kishi S. Tomographic features of a lamellar macular hole formation and a lamellar hole that progressed to a full-thickness macular hole. Am J Ophthalmol. 2000; 130:677–679.
Article21. Liu X, Shen M, Huang S, et al. Repeatability and reproducibility of eight macular intra-retinal layer thicknesses determined by an automated segmentation algorithm using two SD-OCT instruments. PLoS One. 2014; 9:e87996.
Article22. Budenz DL, Fredette MJ, Feuer WJ, Anderson DR. Reproducibility of peripapillary retinal nerve fiber thickness measurements with stratus OCT in glaucomatous eyes. Ophthalmology. 2008; 115:661–666.
Article23. Pinilla I, Garcia-Martin E, Fernandez-Larripa S, et al. Reproducibility and repeatability of Cirrus and Spectralis Fourier-domain optical coherence tomography of healthy and epiretinal membrane eyes. Retina. 2013; 33:1448–1455.
Article24. Park KA, Park DY, Oh SY. Analysis of spectral-domain optical coherence tomography measurements in amblyopia: a pilot study. Br J Ophthalmol. 2011; 95:1700–1706.
Article25. Kim JJ, Im JC, Shin JP, et al. One-year follow-up of macular ganglion cell layer and peripapillary retinal nerve fibre layer thickness changes after panretinal photocoagulation. Br J Ophthalmol. 2014; 98:213–217.
Article26. Garas A, Vargha P, Hollo G. Reproducibility of retinal nerve fiber layer and macular thickness measurement with the RTVue-100 optical coherence tomograph. Ophthalmology. 2010; 117:738–746.
Article27. Ullrich S, Haritoglou C, Gass C, et al. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002; 86:390–393.
Article28. Kusuhara S, Teraoka Escano MF, Fujii S, et al. Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am J Ophthalmol. 2004; 138:709–716.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Repeatability of Retinal Layer Thickness Measurements with Spectral-Domain Optical Coherence Tomography in Normal Eyes
- Analysis of Macular Layer Thickness Measured Using Spectral Domain Optical Coherence Tomography in Korean Subjects
- Comparison of OCT Parameters between the Dominant and Nondominant Eye
- Spectral-Domain Optical Coherence Tomography Findings in Acute Central Retinal Artery Occlusion
- Macular GCIPL Thickness in Idiopathic Macular Telangiectasia Type II