J Korean Ophthalmol Soc.
2009 Apr;50(4):565-571. 10.3341/jkos.2009.50.4.565.
The Effects of Prostaglandin Analogues on the Corneal Thickness
- Affiliations
-
- 1Department of Ophthalmology, St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. jimoon@catholic.ac.kr
- KMID: 2212258
- DOI: http://doi.org/10.3341/jkos.2009.50.4.565
Abstract
-
PURPOSE: To evaluate the effects of prostaglandin analogues on the corneal thickness of patients with primary open-angle glaucoma (POAG) or normal tension glaucoma (NTG).
METHODS
This study included 130 eyes of 65 patients who were diagnosed with POAG or NTG. All patients were divided into two groups; one group received prostaglandin analogues, while the other group received alternative ocular hypotensive eyedrops. Corneal thickness, best corrected visual acuity, and flare in the anterior chamber were measured and compared before treatment and at least 24 months (mean: 27 months) after treatment.
RESULTS
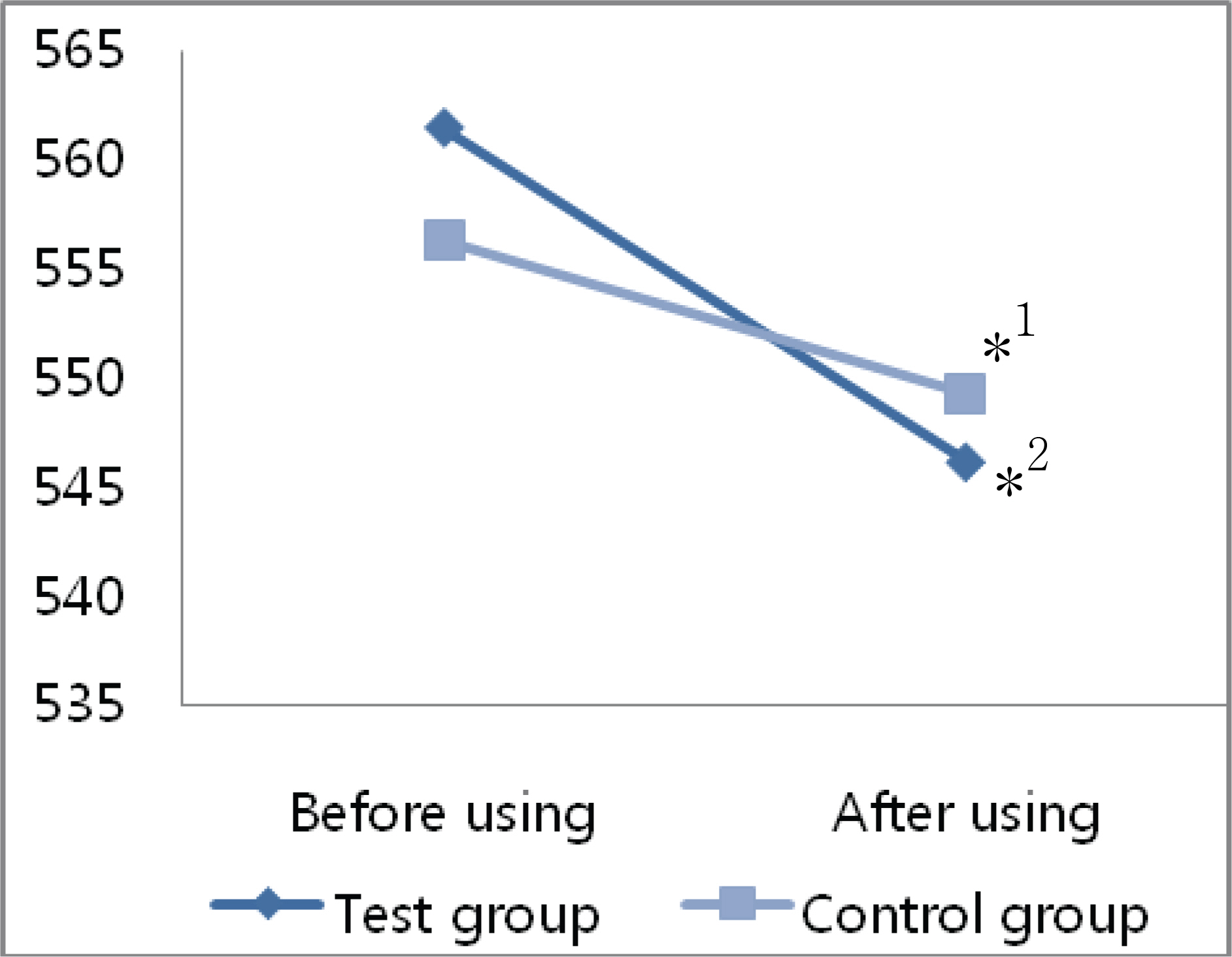
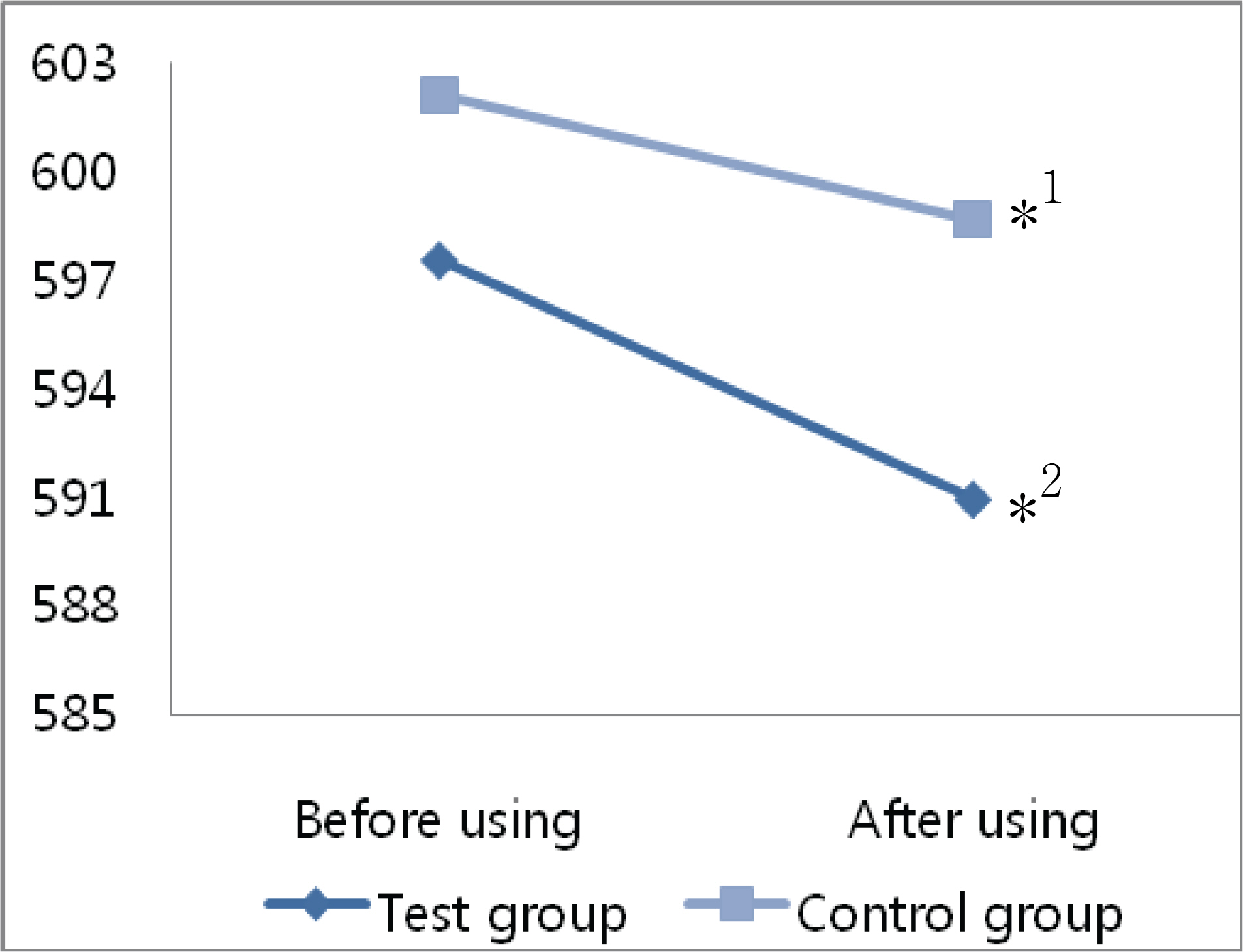
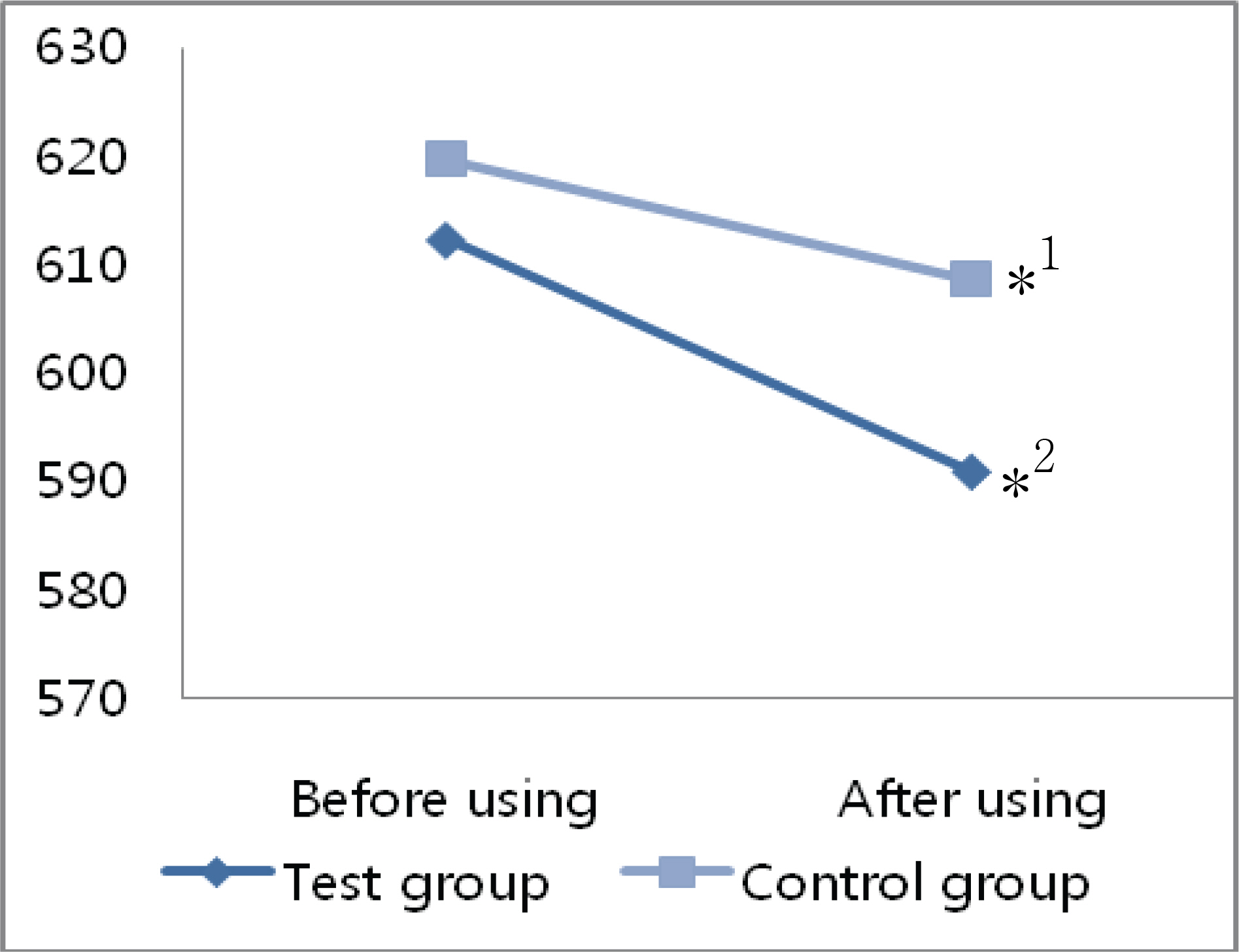
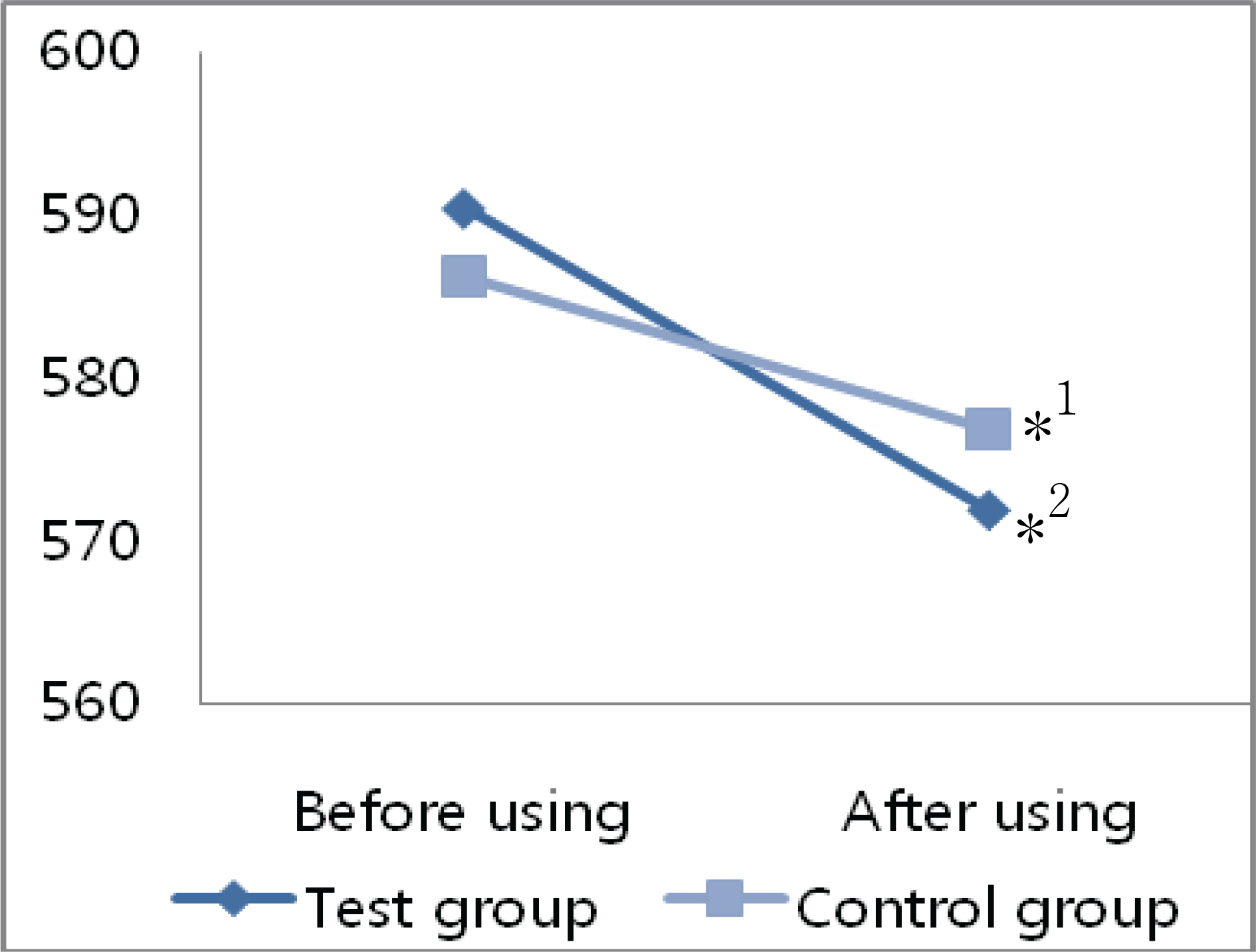
The mean decrease in corneal thickness was statistically significant in the group using prostaglandin analogues, but not in the control group. Among the various prostaglandin analogues used, travoprost and latanoprost decreased mean corneal thickness, but bimatoprost had no effect. Best corrected visual acuity, refraction power, and flare in the anterior chamber did not change significantly in either group of patients when ocular hypotensive eyedrops were used.
CONCLUSIONS
Prostaglandin analogues lower intraocular pressure and decrease corneal thickness if used over a 24 months.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Alexander CL, Miller SJ, Abel SR. Prostaglandin analog treatment of glaucoma and ocular hypotension. Ann Pharmacother. 2002; 36:504–11.2. Wtewart WC, Kolker AE, Stewart JA, et al. conjunctival hyperemia in healthy subjects after short-term dosing with latanoprost, bimatoprost, and travoprost. Am J Ophthalmol. 2003; 135:314–20.3. Camras CB, Toris CB, Tamesis RR. Efficacy and adverse effects of medications used in the treatment of glaucoma. Drugs Aging. 1999; 15:377–88.
Article4. Parrish RK, Palmberg P, Sheu WP. A comparison of latanoprost, bimatoprost, and travoprost in patient with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003; 135:688–703.5. Yousufzai SY, Ye Z, Abdel-Latif AA. Prostaglandin F2 alpha and its analogs induce release of endogenous prostaglandins in iris and ciliary muscles isolated from cat and other mammalian species. Exp Eye Res. 1996; 63:305–10.6. Lass JH, Eriksson GL, Osterling L, Simpson CV. Comparison of the corneal effects of latanoprost, fixed combination latanoprost-timolol, and timolol. Ophthalmology. 2001; 108:264–71.7. Arcieri ES, Pierre Filho PT, Wakamatsu TH, Costa VP. The effects of prostaglandin analogues on the blood aqueous barrier and corneal thickness of phakic patients with primary open-angle glaucoma and ocular hypertension. Eye. 2008; 22:179–83.
Article8. Fanny A, Ouattara A, Coulibaly F, et al. Central corneal thickness and potential error in Goldmann applanation tonometry of the Black African patient suffering from primary open-angle glaucoma: 340 eyes. J Fr Ophtalmol. 2008; 31:405–8.9. Polyer JF, Miller C, Kaufman PL. Prostaglandin F2 alpha effects on isolated rhesus monkey ciliary muscle. Invest Ophthalmol Vis Sci. 1995; 36:2461–5.10. Thieme H, Stumpff F, Ottlecz A, et al. Mechanisms of action of unoprostone on trabecular meshwork contractility. Invest Ophthalmol Vis Sci. 2001; 42:3193–201.11. Lindsey JD, Kashiwagi K, Kashiwagi F, Weinreb RN. Prostaglandins alter extracellular matrix adjacent to human ciliary muscle cells in vitro. Invest Ophthalmol Vis Sci. 1997; 38:2214–23.12. Lindsey JD, Kashiwagi K, Kashiwagi F, Weinreb RN. Prostaglandin action on ciliary smooth muscle extracelluar matrix metabolism: implications for uveoscleral outflow. Surv Ophthalmol. 1997; 41:S53–9.13. Kashiwagi K, Jin M, Suzuki M, et al. Isopropyl unoprostone increases the activities of matrix metalloproteinases in cultured monkey ciliary muscle cells. J Glaucoma. 2001; 10:271–6.
Article14. Collier SA, Madigan MC, Penfold PL. Expression of membrane-type 1 matrix metalloproteinase (MT1-MMP) and MMP-2 in normal and keratoconus corneas. Curr Eye Res. 2000; 21:662–8.
Article15. Collier SA. Is the corneal degradation in keratoconus caused by matrix-metallloproteinases? Clin Experiment Ophthalmol. 2001; 29:340–4.16. Harasymowycz PJ, Papamatheakis DG, Ennis M, et al. Relationship between travoprost and central corneal thickness in ocular hypertension and open-angle glaucoma. Cornea. 2007; 26:34–41.
Article17. Brandt JD, Gordon MO, Beiser JA, et al. Changes in Central corneal thickness over time: the ocular hypertension treatment study. Ophthalmology. 2008; 115:1550–6.18. Woodward DF, Krauss AH, Chen J, et al. Pharmacological characterization of a novel antiglaucoma agent, Bimatoprost (AGN 192024). J Pharmacol Exp Ther. 2003; 305:772–85.
Article19. Woodward DF, Krauss AH, Chen J, et al. The pharmacology of bimatoprost (Lumigan). Surv Ophthalmol. 2001; 45:337–45.
Article20. Maxey KM, Johnson JL, LaBrecque J. The hydrolysis of bimatoprost in corneal tissue generates a potent prostanoid FP receptor agonist. Surv Ophthalmol. 2002; 47:34–40.
Article21. von Bahr G. Measurements of the thickness of the cornea. Acta Ophthalmol (Copenh). 1948; 26:247–66.22. Hansen FK. A clinical study of the normal human central corneal thickness. Acta Ophthalmol (Copenh). 1971; 49:82–9.23. Wolfs RC, Klaver CC, Vingerling JR, et al. Distribution of central corneal thickness and its association with intraocular pressure: the Rotterdam Study. Am J Ophthalmol. 1997; 123:767–72.
Article24. Brandt JD, Beiser JA, Kass MA, et al. Central corneal thickness in the Ocular hypertension Treatment Study (OHTS). Ophthalmology. 2001; 108:1779–88.
Article25. Hahn S, Azen S, Ying-Lai M, et al. Central corneal thickness in Latinos. Invest Ophthalmol Vis Sci. 2003; 44:1508–12.
Article26. Kang PS, Yang YS, Kim JD. Comparison of corneal thickness measurements with the orbscan and ultrasonic pachymetry. J Korean Ophthalmol Soc. 2000; 41:1697–703.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Short-Term Effect of Prostaglandin Analog Monotherapy on Corneal Biomechanical Properties in Normal Tension Glaucoma Patients
- Effect of Prostaglandin Analogues on Central Corneal Thickness: 3-Year Follow-up Results
- Topical Prostaglandin Analogue Drugs Inhibit Adipocyte Differentiation
- The Long-term Effects of Prostaglandin Analogues on Central Corneal Thickness and Intraocular Pressure
- Effect of the Preservative Benzalkonium Chloride in Prostaglandin Analogues on Corneal Sensitivity