J Periodontal Implant Sci.
2010 Jun;40(3):132-138. 10.5051/jpis.2010.40.3.132.
Bone regeneration effects of human allogenous bone substitutes: a preliminary study
- Affiliations
-
- 1Department of Periodontology and Dental Research Institute, Seoul National University College of Dentistry, Seoul, Korea. periopf@snu.ac.kr
- KMID: 2212140
- DOI: http://doi.org/10.5051/jpis.2010.40.3.132
Abstract
- PURPOSE
The purpose of this study was to compare the bone regeneration effects of cortical, cancellous, and cortico-cancellous human bone substitutes on calvarial defects of rabbits.
METHODS
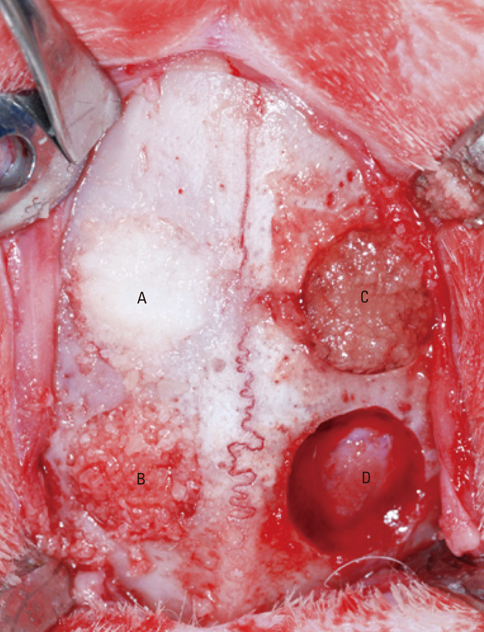
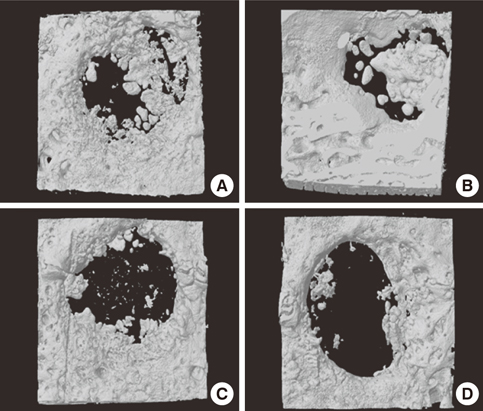
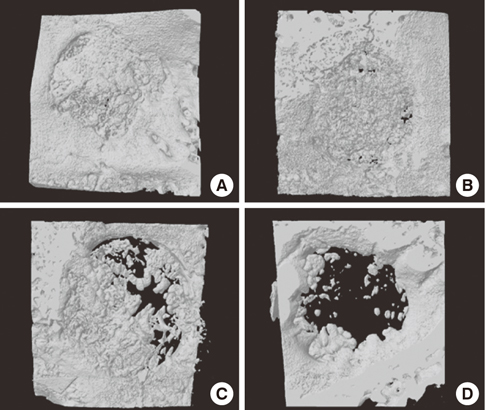
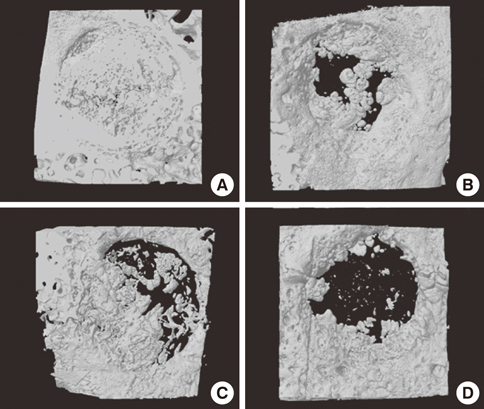
Four 8-mm diameter calvarial defects were created in each of nine New Zealand white rabbits. Freeze-dried cortical bone, freeze-dried cortico-cancellous bone, and demineralized bone matrix with freeze-dried cancellous bone were inserted into the defects, while the non-grafted defect was regarded as the control. After 4, 8, and 12 weeks of healing, the experimental animals were euthanized for specimen preparation. Micro-computed tomography (micro-CT) was performed to calculate the percent bone volume. After histological evaluation, histomorphometric analysis was performed to quantify new bone formation.
RESULTS
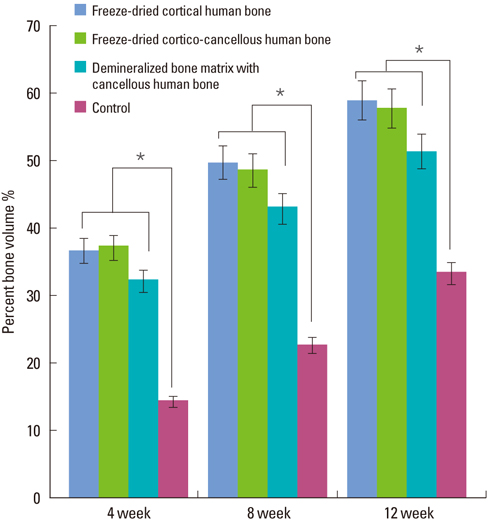
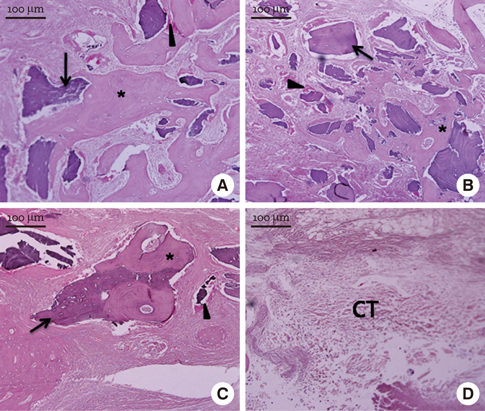
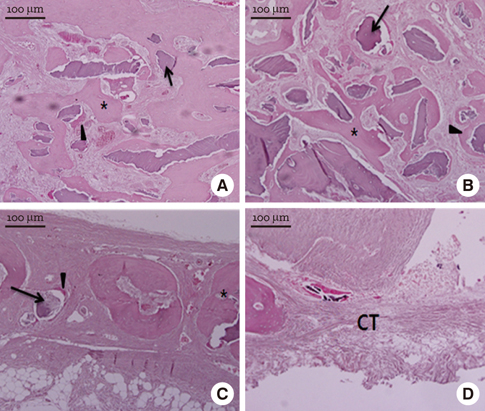
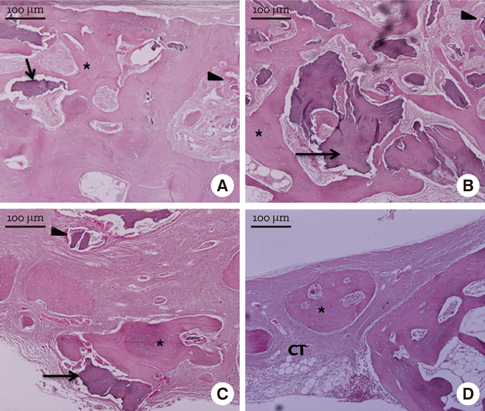
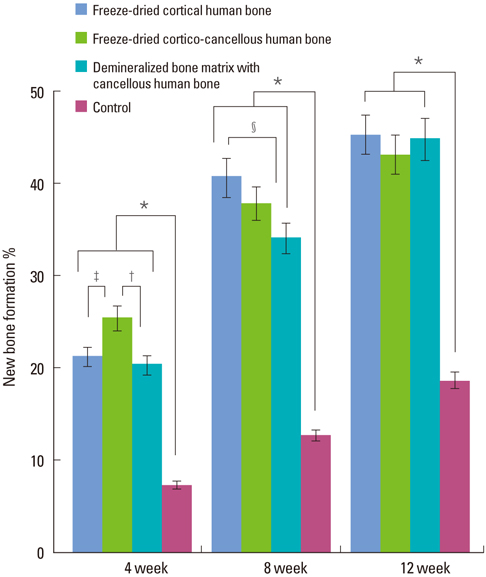
In micro-CT evaluation, freeze-dried cortico-cancellous human bone showed the highest percent bone volume value among the experimental groups at week 4. At week 8 and week 12, freeze-dried cortical human bone showed the highest percent bone volume value among the experimental groups. In histologic evaluation, at week 4, freeze-dried cortico-cancellous human bone showed more prominent osteoid tissue than any other group. New bone formation was increased in all of the experimental groups at week 8 and 12. Histomorphometric data showed that freeze-dried cortico-cancellous human bone showed a significantly higher new bone formation percentile value than any other experimental group at week 4. At week 8, freeze-dried cortical human bone showed the highest value, of which a significant difference existed between freeze-dried cortical human bone and demineralized bone matrix with freeze-dried cancellous human bone. At week 12, there were no significant differences among the experimental groups.
CONCLUSIONS
Freeze-dried cortico-cancellous human bone showed swift new bone formation at the 4-week healing phase, whereas there was less difference in new bone formation among the experimental groups in the following healing phases.
MeSH Terms
Figure
Reference
-
1. Helm GA, Dayoub H, Jane JA Jr. Bone graft substitutes for the promotion of spinal arthrodesis. Neurosurg Focus. 2001; 10:E4.
Article2. Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005; 36:Suppl 3. S20–S27.
Article3. Urist MR. Bone: formation by autoinduction. Science. 1965; 150:893–899.
Article4. Misch CE, Dietsh F. Bone-grafting materials in implant dentistry. Implant Dent. 1993; 2:158–167.
Article5. Pietrzak WS, Woodell-May J, McDonald N. Assay of bone morphogenetic protein-2, -4, and -7 in human demineralized bone matrix. J Craniofac Surg. 2006; 17:84–90.
Article6. Mellonig JT, Bowers GM, Bailey RC. Comparison of bone graft materials. Part I. New bone formation with autografts and allografts determined by Strontium-85. J Periodontol. 1981; 52:291–296.
Article7. Mellonig JT, Bowers GM, Cotton WR. Comparison of bone graft materials. Part II. New bone formation with autografts and allografts: a histological evaluation. J Periodontol. 1981; 52:297–302.
Article8. Piattelli A, Scarano A, Corigliano M, Piattelli M. Comparison of bone regeneration with the use of mineralized and demineralized freeze-dried bone allografts: a histological and histochemical study in man. Biomaterials. 1996; 17:1127–1131.
Article9. Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1989; 60:655–663.
Article10. Mellonig JT, Bowers GM, Bright RW, Lawrence JJ. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects. J Periodontol. 1976; 47:125–131.
Article11. Cammack GV 2nd, Nevins M, Clem DS 3rd, Hatch JP, Mellonig JT. Histologic evaluation of mineralized and demineralized freeze-dried bone allograft for ridge and sinus augmentations. Int J Periodontics Restorative Dent. 2005; 25:231–237.12. Cornell CN. Osteobiologics. Bull Hosp Jt Dis. 2004; 62:13–17.13. Behairy Y, Jasty M. Bone grafts and bone substitutes in hip and knee surgery. Orthop Clin North Am. 1999; 30:661–671.
Article14. Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop Relat Res. 1987; 7–16.
Article15. Ludwig SC, Boden SD. Osteoinductive bone graft substitutes for spinal fusion: a basic science summary. Orthop Clin North Am. 1999; 30:635–645.16. Schreurs BW, Slooff TJ, Buma P, Gardeniers JW, Huiskes R. Acetabular reconstruction with impacted morsellised cancellous bone graft and cement. A 10- to 15-year follow-up of 60 revision arthroplasties. J Bone Joint Surg Br. 1998; 80:391–395.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The comparative study of bone substitute materials in bone regeneration
- Biomaterial development for oral and maxillofacial bone regeneration
- Hard tissue regeneration using bone substitutes: an update on innovations in materials
- Bone Substitutes and the Advancement for Enhancing Bone Healing
- Effect of deproteinized bovine bone mineral on cell proliferation in the procedure of guided bone regeneration