J Pathol Transl Med.
2016 Jan;50(1):10-16. 10.4132/jptm.2015.09.15.
Menetrier's Disease: Its Mimickers and Pathogenesis
- Affiliations
-
- 1Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA. kay.washington@vanderbilt.edu
- 2Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
- 3Department of Veterans Affairs Medical Center, Nashville, TN, USA.
- KMID: 2211396
- DOI: http://doi.org/10.4132/jptm.2015.09.15
Abstract
- Menetrier's disease is a rare protein-losing hypertrophic gastropathy. Histologically, it can be mistaken for other disorders showing hypertrophic gastropathy. The pathogenesis of Menetrier's disease is not fully understood; however, it appears that the epidermal growth factor receptor (EGFR) ligand, transforming growth factor alpha, contributes to the pathogenesis of this disorder. In this review, we will discuss disease entities that can mimic Menetrier's disease and the role of EGFR signaling in Menetrier's disease.
MeSH Terms
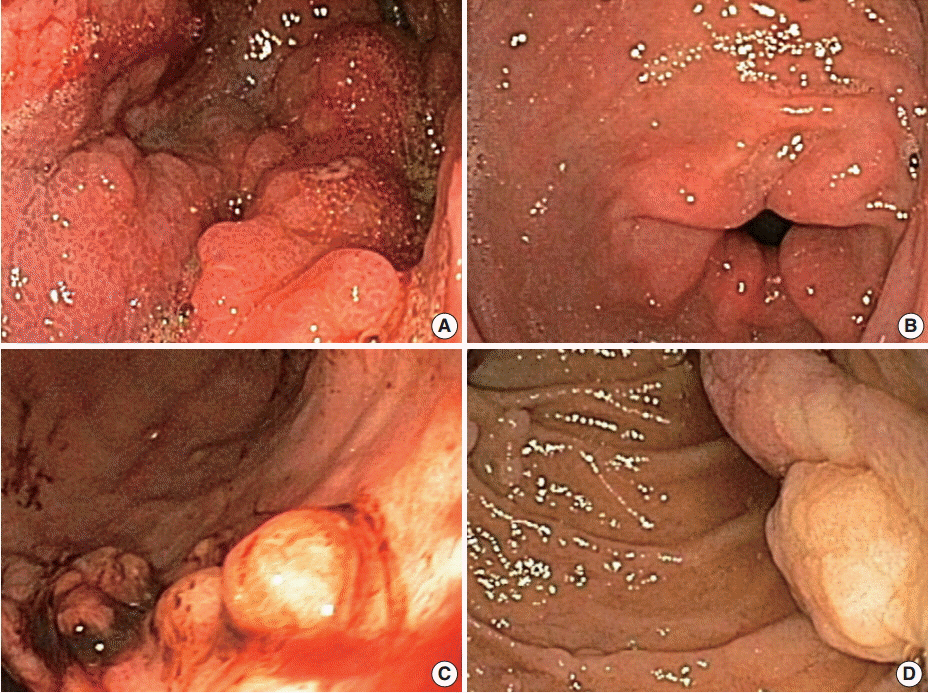
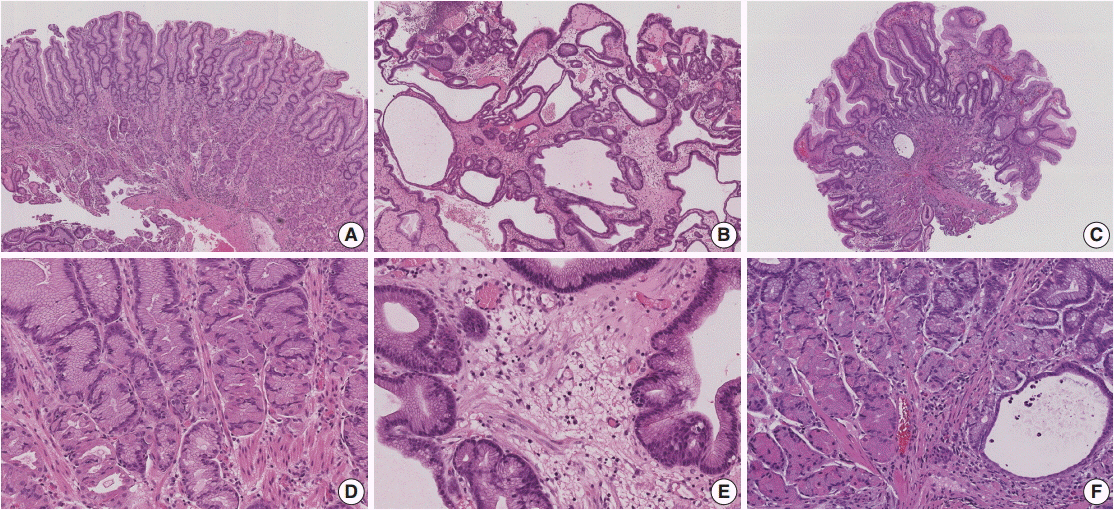
Figure
Reference
-
1. Ménétrier P. Des polyadenomes gastriques et de leurs rapport avec le cancer de l’estomac. Arch Physiol Norm Pathol. 1888; 1:236–62.2. Scharschmidt BF. The natural history of hypertrophic gastrophy (Ménétrier’s disease): report of a case with 16 year follow-up and review of 120 cases from the literature. Am J Med. 1977; 63:644–52.3. Coffey RJ, Washington MK, Corless CL, Heinrich MC. Ménétrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J Clin Invest. 2007; 117:70–80.
Article4. Eisenstat DD, Griffiths AM, Cutz E, Petric M, Drumm B. Acute cytomegalovirus infection in a child with Ménétrier’s disease. Gastroenterology. 1995; 109:592–5.
Article5. Hoffer V, Finkelstein Y, Balter J, Feinmesser M, Garty BZ. Ganciclovir treatment in Ménétrier’s disease. Acta Paediatr. 2003; 92:983–5.
Article6. Badov D, Lambert JR, Finlay M, Balazs ND. Helicobacter pylori as a pathogenic factor in Ménétrier’s disease. Am J Gastroenterol. 1998; 93:1976–9.7. Drut RM, Gomez MA, Lojo MM, Drut R. Cytomegalovirus-associated Ménétrier’s disease in adults: demonstration by polymerase chain reaction (PCR). Medicina (B Aires). 1995; 55:659–64.8. Rich A, Toro TZ, Tanksley J, et al. Distinguishing Ménétrier’s disease from its mimics. Gut. 2010; 59:1617–24.
Article9. Setakhr V, Muller G, Hoang P, Lambert AS, Geubel A. Cytomegalovirus-associated protein losing gastropathy in an immunocompetent adult: a case report. Acta Gastroenterol Belg. 2007; 70:296–9.10. Xiao SY, Hart J. Marked gastric foveolar hyperplasia associated with active cytomegalovirus infection. Am J Gastroenterol. 2001; 96:223–6.
Article11. Fiske WH, Tanksley J, Nam KT, et al. Efficacy of cetuximab in the treatment of Ménétrier’s disease. Sci Transl Med. 2009; 1:8ra18.
Article12. Hatemi I, Caglar E, Atasoy D, Goksel S, Dobrucali A. Ménétrier’s disease coexisting with ulcerative colitis and sclerosing cholangitis. Dig Liver Dis. 2008; 40:78–9.13. Wolfsen HC, Carpenter HA, Talley NJ. Ménétrier’s disease: a form of hypertrophic gastropathy or gastritis? Gastroenterology. 1993; 104:1310–9.14. Stempien SJ, Dagradi AE, Reingold IM, et al. Hypertrophic hypersecretory gastropathy: analysis of 15 cases and a review of the pertinent literature. Am J Dig Dis. 1964; 9:471–93.15. Tan DT, Stempien SJ, Dagradi AE. The clinical spectrum of hypertrophic hypersecretory gastropathy: report of 50 patients. Gastrointest Endosc. 1971; 18:69–73.16. Carmack SW, Genta RM, Schuler CM, Saboorian MH. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. 2009; 104:1524–32.
Article17. Pintiliciuc OG, Heresbach D, de-Lajarte-Thirouard AS, et al. Gastric involvement in juvenile polyposis associated with germline SMAD4 mutations: an entity characterized by a mixed hypertrophic and polypoid gastropathy. Gastroenterol Clin Biol. 2008; 32(5 pt 1):445–50.18. Cronkhite LW Jr, Canada WJ. Generalized gastrointestinal polyposis; an unusual syndrome of polyposis, pigmentation, alopecia and onychotrophia. N Engl J Med. 1955; 252:1011–5.19. Lam-Himlin D, Park JY, Cornish TC, Shi C, Montgomery E. Morphologic characterization of syndromic gastric polyps. Am J Surg Pathol. 2010; 34:1656–62.
Article20. Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013; 105:1607–16.
Article21. Coriat R, Mozer M, Caux F, et al. Endoscopic findings in Cowden syndrome. Endoscopy. 2011; 43:723–6.
Article22. Worthley DL, Phillips KD, Wayte N, et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut. 2012; 61:774–9.
Article23. Yanaru-Fujisawa R, Nakamura S, Moriyama T, et al. Familial fundic gland polyposis with gastric cancer. Gut. 2012; 61:1103–4.
Article24. Dempsey PJ, Goldenring JR, Soroka CJ, et al. Possible role of transforming growth factor alpha in the pathogenesis of Ménétrier’s disease: supportive evidence form humans and transgenic mice. Gastroenterology. 1992; 103:1950–63.25. Matsui Y, Halter SA, Holt JT, Hogan BL, Coffey RJ. Development of mammary hyperplasia and neoplasia in MMTV-TGF alpha transgenic mice. Cell. 1990; 61:1147–55.26. Takagi H, Fukusato T, Kawaharada U, Kuboyama S, Merlino G, Tsutsumi Y. Histochemical analysis of hyperplastic stomach of TGF-alpha transgenic mice. Dig Dis Sci. 1997; 42:91–8.27. Takagi H, Jhappan C, Sharp R, Merlino G. Hypertrophic gastropathy resembling Ménétrier’s disease in transgenic mice overexpressing transforming growth factor alpha in the stomach. J Clin Invest. 1992; 90:1161–7.
Article28. Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990; 61:1121–35.29. Bluth RF, Carpenter HA, Pittelkow MR, Page DL, Coffey RJ. Immunolocalization of transforming growth factor-alpha in normal and diseased human gastric mucosa. Hum Pathol. 1995; 26:1333–40.30. Romano M, Meise KS, Suozzo R, Sessa G, Persico M, Coffey RJ. Regional distribution of transforming growth factor-alpha and epidermal growth factor in normal and portal hypertensive gastric mucosa in humans. Dig Dis Sci. 1995; 40:263–7.
Article31. Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990; 61:1137–46.32. Singh B, Coffey RJ. Trafficking of epidermal growth factor receptor ligands in polarized epithelial cells. Annu Rev Physiol. 2014; 76:275–300.
Article33. Singh B, Coffey RJ. From wavy hair to naked proteins: the role of transforming growth factor alpha in health and disease. Semin Cell Dev Biol. 2014; 28:12–21.
Article34. Coffey RJ Jr, Tanksley J. Pierre Ménétrier and his disease. Trans Am Clin Climatol Assoc. 2012; 123:126–33.35. Sharp R, Babyatsky MW, Takagi H, et al. Transforming growth factor alpha disrupts the normal program of cellular differentiation in the gastric mucosa of transgenic mice. Development. 1995; 121:149–61.
Article36. Bockman DE, Sharp R, Merlino G. Regulation of terminal differentiation of zymogenic cells by transforming growth factor alpha in transgenic mice. Gastroenterology. 1995; 108:447–54.37. Goldenring JR, Ray GS, Soroka CJ, et al. Overexpression of transforming growth factor-alpha alters differentiation of gastric cell lineages. Dig Dis Sci. 1996; 41:773–84.38. Nomura S, Settle SH, Leys CM, et al. Evidence for repatterning of the gastric fundic epithelium associated with Ménétrier’s disease and TGFalpha overexpression. Gastroenterology. 2005; 128:1292–305.39. Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003; 424:456–61.
Article40. Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004; 303:848–51.41. Cheng N, Bhowmick NA, Chytil A, et al. Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks. Oncogene. 2005; 24:5053–68.42. Shinohara M, Mao M, Keeley TM, et al. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010; 139:2050–60. e2.
Article43. Suter WR, Neuweiler J, Borovicka J, Binek J, Fantin AC, Meyenberger C. Cytomegalovirus-induced transient protein-losing hypertrophic gastropathy in an immunocompetent adult. Digestion. 2000; 62:276–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Co-Infection with Cytomegalovirus and Helicobacter pylori in a Child with Menetrier's Disease
- Menetrier's disease accompanied thrombosis of the iliac vein: A case report
- A Case of Menetrier's Disease
- A Case of Menetrier's Disease Associated with Two Adenomatous Polyps
- Menetrier's Disease Report of two cases