J Korean Soc Med Inform.
2009 Jun;15(2):235-244. 10.4258/jksmi.2009.15.2.235.
Wireless Clinical Trial of Data Capture using a Personal Digital Assistant
- Affiliations
-
- 1Division of Vaccine Preventable Disease Control & National Immunization Program, Department of Infectious Disease Control, Korea.
- 2Department of Internal Medicine, St. Mary Hospital, The Catholic University of Korea, Korea.
- 3College of Information Sciences, Hansin University, Korea.
- 4Graduate School of Public Health, Yonsei University, Korea.
- 5Department of Preventive Medicine, College of Medicine, The Catholic University of Korea, Korea.
- 6Catholic Medical Center, Clinical Research Coordinating Center, The Catholic University of Korea, Korea. iychoi@catholic.ac.kr
- KMID: 2211393
- DOI: http://doi.org/10.4258/jksmi.2009.15.2.235
Abstract
OBJECTIVE
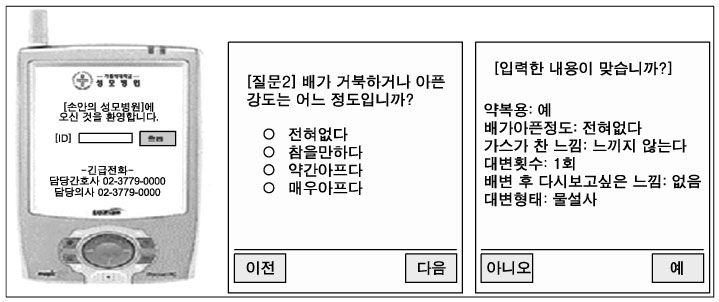
Personal Digital Assistants (PDAs) have the potential to improve clinical trial data collection; however, most current PDA-based clinical data collection systems typically collect and store data in the offline mode, and then transfer the data to an operational database. The purpose of this study was to explore the usefulness of a wireless clinical data collection system for an irritable bowel syndrome trial compared with the traditional paper based data collection. METHODS: We have developed a PDA-based data capture system for clinical trials, and tested it in a double-blind trial. Sixty four patients with irritable bowel syndrome were randomly selected and divided into a control group that used the standard paper report forms (CRF) and an intervention group that used the electronic report forms (e-CRF), daily for five weeks. There were 630 data sets consisting of six questions each, and thus 3,570 data points total were collected. RESULTS: The response rate of the control group was significantly higher than that of the intervention group. However, the completeness of the response in the intervention group was higher and the number of input errors per person for the PDA group was lower than in the paper group. CONCLUSION: A PDA based electronic diary improved the response rate and decreased input errors in an IBS trial. We conclude that mobile devices can be very useful, especially when the proposed design and connectivity aspects have been taken into account.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Development of u-House Gateway for u-Healthcare
Dongwoo Ro, Sooyoung Yoo, Kyungwoo Cheon, Jinwook Choi
J Korean Soc Med Inform. 2009;15(4):465-474. doi: 10.4258/jksmi.2009.15.4.465.
Reference
-
1. Welker JA. Implementation of electronic data capture systems: barriers and solutions. Contemp Clin Trials. 2007. 28(3):329–336.
Article2. Marks RG. Validating electronic source data in clinical trials. Control Clin Trials. 2004. 25(5):437–446.
Article3. Bunn G. Scaling up EDC: How to move away from paper trials. Appl Clin Trials. 2002. SDC Suppl. (1):2–4.4. Santoro E, Nicolis E, Franzosi MG, Tognoni G. Internet for clinical trials past, present, and future. Controlled Clinical Trials. 1999. 20(2):194–201.5. Kelly MA, Oldham J. The Internet and randomised controlled trials. International Journal of Medical Informatics. 1997. 47(1-2):91–99.
Article6. Schrezenmeir J, Achterberg H, Bergeler J, Kustner E, Stumer W, Hutten H, et al. Controlled study on the use of hand-held insulin dosage computers enabling conversion to and optimizing of meal-related insulin therapy regimens. Life Support Syst. 1985. 3(1):561–567.7. Clarke WL, Cox DJ, Gonder-Frederick LA, Kovatchev B. Hypoglycemia and the decision to drive a motor vehicle by persons with diabetes. J Am Med Assoc. 1999. 282:750–754.
Article8. Greeno CG, Wing RR, Shiffman S. Binge antecedents in obese women with and without binge eating disorder. J Consult Clin Psychol. 2000. 68(1):95–102.
Article9. McBride JS, Anderson RT, Bahnson JL. Using a hand-held computer to collect data in an orthopedic outpatient clinic: a randomized trial of two survey methods. Medical Care. 1999. 37(7):647.
Article10. Koop A, Mösges R. The use of handheld computers in clinical trials. Controlled Clinical Trials. 2002. 23(5):469–480.
Article11. Tsang MW, Mok M, Kam G, Jung M, Tang A, Chan U. Improvement in diabetes control with a monitoring system based on a handheld, touchscreen electronic diary. Journal of Telemedicine and Telecare. 2001. 7(1):47.
Article12. Duftschmid G, Gall W, Eigenbauer E, Dorda W. Management of data from clinical trials using the ArchiMed system. Medical Informatics and the Internet in Medicine. 2002. 27(2):85–98.
Article13. Beinlich I, Bokemeyer C, RäTh U, Walter-Kirst R, Hartlapp J, Muschiol J, et al. Pen-based remote data entry system: a pilot clinical trial. Arzneimittel-Forschun. 1993. 43(3):399–404.14. Reilly JC, Wallace M, Campbell MM. Tracking pharmacist interventions with a hand-held computer. American Journal of Health-System Pharmacy. 2001. 58(2):158.
Article15. Carroll AE, Tarczy-Hornoch P, O'Reilly E, Christakis DA. The effect of point-of-care personal digital assistant use on resident documentation discrepancies. Pediatrics. 2004. 113(3):450–454.
Article16. Jao C, Hier DB, Su J, editors. Evaluating a Digital Resident Diagnosis Log: Reasons for Limited Acceptance of a PDA Solution. 2003. American Medical Informatics Association.17. Keshavjee K, Lawson ML, Malloy M, Hubbard S, Grass M, editors. Technology Failure Analysis: Understanding Why A Diabetes Management Tool Developed for A Personal Digital Assistant (PDA) Didn't Work in a Randomized Control Trial. 2003. American Medical Informatics Association.18. Kush RD. Eclinical trials: planning and implementation. 2003. 1st ed. Boston: Centerwatch Inc;15–35.19. Bang J, Choi I. Dholakia N, Rask M, Dholakia RR, editors. South Korea: The emerging tech notopia. M-commerce: global experiences and perspectives. 2006. 1st ed. Hershey: Idea Group Pub.;197–219.20. Walker LS, Sorrells S. Brief report: assessment of children's gastrointestinal symptoms for clinical trials. Journal of Pediatric Psychology. 2002. 27(3):303.
Article21. Matthew AG, Currie KL, Irvine J, Ritvo P, Santa Mina D, Jamnicky L, et al. Serial personal digital assistant data capture of health-related quality of life: a randomized controlled trial in a prostate cancer clinic. Health and Quality of Life Outcomes. 2007. 5(1):38.
Article22. Lal SO, Smith FW, Davis JP, Castro HY, Smith DW, Chinkes DL, et al. Palm computer demonstrates a fast and accurate means of burn data collection. The Journal of Burn Care. Rehabilitation. 2000. 21(6):559–561.
Article23. Hyland ME, Kenyon CA, Allen R, Howarth P. Diary keeping in asthma: comparison of written and electronic methods. BMJ: British Medical Journal. 1993. 306(6876):487.
Article24. Kos J, Battig K. Comparison of an electronic food diary with a nonquantitative food frequency questionnaire in male and female smokers and nonsmokers. Journal of the American Dietetic Association. 1996. 96(3):283–285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Home Hospice Information SystemUsing Personal Digital Assistants: In Association with the Goryeong Health Center
- A Review of Assistive Listening Device and Digital Wireless Technology for Hearing Instruments
- Development and Application of Direct Data Capture for Monitoring Medication Compliance in Clinical Trials
- The Remote Health System utilizing Wireless Communication of Personal Health Information
- Personal digital assistants: Essential tools for preparing dietetics professionals to use new generation information technology