Healthc Inform Res.
2017 Oct;23(4):249-254. 10.4258/hir.2017.23.4.249.
Development and Application of Direct Data Capture for Monitoring Medication Compliance in Clinical Trials
- Affiliations
-
- 1Department of Clinical Pharmacology, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea. eykim@inje.ac.kr
- KMID: 2396415
- DOI: http://doi.org/10.4258/hir.2017.23.4.249
Abstract
OBJECTIVES
The monitoring of medication compliance in clinical trials is important but labor intensive. To check medication compliance in clinical trials, a system was developed, and its technical feasibility evaluated.
METHODS
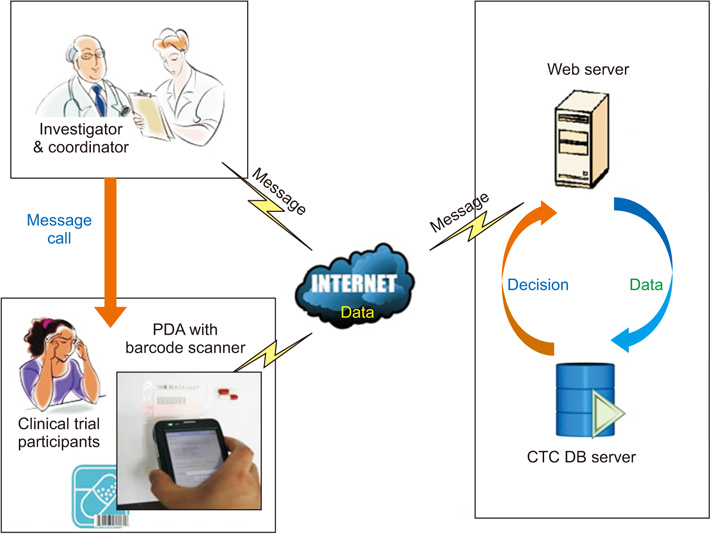
The system consisted of three parts: a management part (clinical trial center database and a developed program), clinical trial investigator part (monitoring), and clinical trial participant part (personal digital assistant [PDA] with a barcode scanner). The system was tested with 20 participants for 2 weeks, and compliance was evaluated.
RESULTS
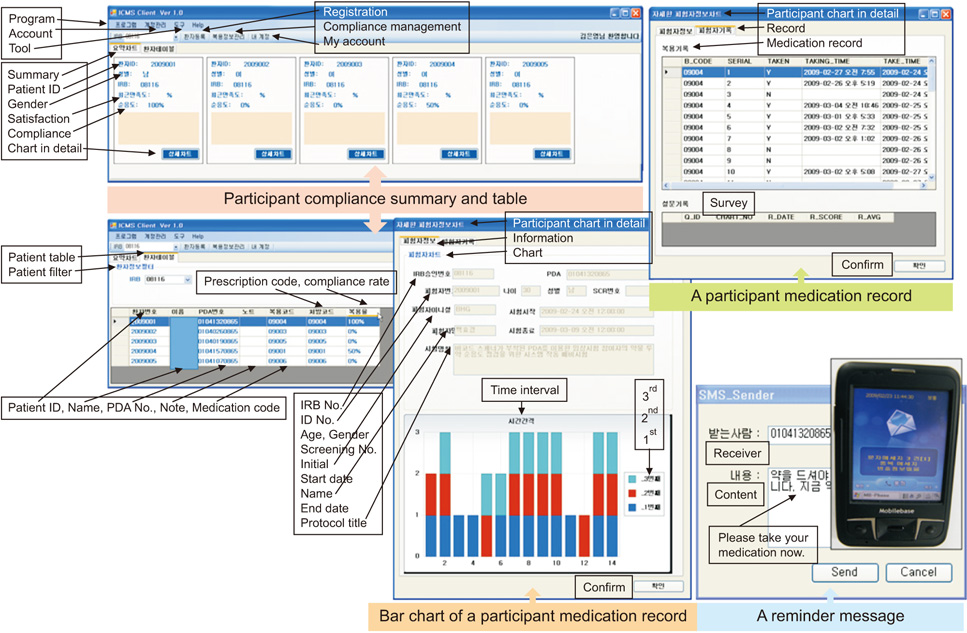
This study developed a medication compliance monitoring system that used a PDA with a barcode scanner, which sent reminder/warning messages, logged medication barcode data, and provided compliance information to investigators. Registered participants received short message service (SMS) reminder/warning messages on their PDA and sent barcode data at the dosing time. The age range of the participants was 29 to 73 years. Five participants were <50 years old and 8 were ≥65 years old. The total mean compliance rate was 82.3%. The mean compliance rate was 83.1% in participants <65 years old and 81.1% in those ≥65 years old.
CONCLUSIONS
The system was feasible, usable, and effective, even with elderly participants, for monitoring medication compliance in clinical trials using a PDA with a barcode scanner, and may improve the quality of clinical trials.
Keyword
MeSH Terms
Figure
Reference
-
1. Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (Weltel Kenya1): a randomised trial. Lancet. 2010; 376(9755):1838–1845.
Article2. Pernell BM, DeBaun MR, Becker K, Rodeghier M, Bryant V, Cronin RM. Improving medication adherence with two-way short message service reminders in sickle cell disease and asthma: a feasibility randomized controlled trial. Appl Clin Inform. 2017; 8(2):541–559.3. Huang CY, Nguyen PA, Clinciu DL, Hsu CK, Lu JR, Yang HC, et al. A personalized medication management platform (PMMP) to improve medication adherence: a randomized control trial. Comput Methods Programs Biomed. 2017; 140:275–281.
Article4. van Dam J, Omondi Onyango K, Midamba B, Groosman N, Hooper N, Spector J, et al. Open-source mobile digital platform for clinical trial data collection in lowresource settings. BMJ Innov. 2017; 3(1):26–31.
Article5. Andriesen J, Bull S, Dietrich J, Haberer JE, Van Der Pol B, Voronin Y, et al. Using digital technologies in clinical hiv research: Real-world applications and considerations for future work. J Med Internet Res. 2017; 19(7):e274.
Article6. Welker JA. Implementation of electronic data capture systems: barriers and solutions. Contemp Clin Trials. 2007; 28(3):329–336.
Article7. Turner D. Digital data capture for electronic patient record systems. J AHIMA. 1995; 66(3):63–65.8. Bushnell DM, Reilly MC, Galani C, Martin ML, Ricci JF, Patrick DL, et al. Validation of electronic data capture of the irritable bowel syndrome: quality of life measure, the work productivity and activity impairment questionnaire for irritable bowel syndrome and the EuroQol. Value Health. 2006; 9(2):98–105.
Article9. Nyholm D, Kowalski J, Aquilonius SM. Wireless realtime electronic data capture for self-assessment of motor function and quality of life in Parkinson's disease. Mov Disord. 2004; 19(4):446–451.
Article10. Boissy P, Jacobs K, Roy SH. Usability of a barcode scanning system as a means of data entry on a PDA for selfreport health outcome questionnaires: a pilot study in individuals over 60 years of age. BMC Med Inform Decis Mak. 2006; 6:42.
Article11. Evans D, Berhanu R, Moyo F, Nguweneza A, Long L, Fox MP. Can short-term use of electronic patient adherence monitoring devices improve adherence in patients failing second-line antiretroviral therapy? Evidence from a pilot study in Johannesburg, South Africa. AIDS Behav. 2016; 20(11):2717–2728.
Article12. Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999; 21(6):1074–1090.
Article13. Kalantarian H, Motamed B, Alshurafa N, Sarrafzadeh M. A wearable sensor system for medication adherence prediction. Artif Intell Med. 2016; 69:43–52.
Article14. Kalantarian H, Alshurafa N, Sarrafzadeh M. Detection of gestures associated with medication adherence using smartwatch-based inertial sensors. IEEE Sens J. 2016; 16(4):1054–1061.
Article15. Story A, Garfein RS, Hayward A, Rusovich V, Dadu A, Soltan V, et al. Monitoring therapy compliance of tuberculosis patients by using video-enabled electronic devices. Emerg Infect Dis. 2016; 22(3):538–540.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Predicting Medication Compliance among Elderly Visitors of Public Health Centers
- Issues of Compliance in Osteoporosis Medication
- Development of a Structural Model Explaining Medication Compliance of Persons with Schizophrenia
- The Effects of Group Motivational Interviewing Compliance Therapy on Drug Attitude, Medicine Application Self-efficacy and Medicine Application in Psychiatric Patients
- Research Development Using REDCap Software