J Korean Soc Radiol.
2010 Mar;62(3):235-243. 10.3348/jksr.2010.62.3.235.
Multidetector CT Assessment of Lymph Node Size for Nodal Staging in Patients with Potentially Operable Squamous Esophageal Cancer and the 18F-FDG Positron Emission Tomography CT Correlation
- Affiliations
-
- 1Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Korea. jijung@catholic.ac.kr
- 2Department of Radiology, Gyeongsang National University Hospital, Korea.
- 3Department of Radiology, St. Vincent Hospital, College of Medicine, The Catholic University of Korea, Korea.
- 4Department of Thoracic Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Korea.
- 5Department of Nuclear Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Korea.
- KMID: 2208918
- DOI: http://doi.org/10.3348/jksr.2010.62.3.235
Abstract
- PURPOSE
To investigate the size criteria of multidetector computed tomography (MDCT) for the evaluation metastatic lymph nodes (LNs) for potentially operable squamous esophageal cancer, and to compare this information with the results of positron emission tomography-CT (PET-CT).
MATERIALS AND METHODS
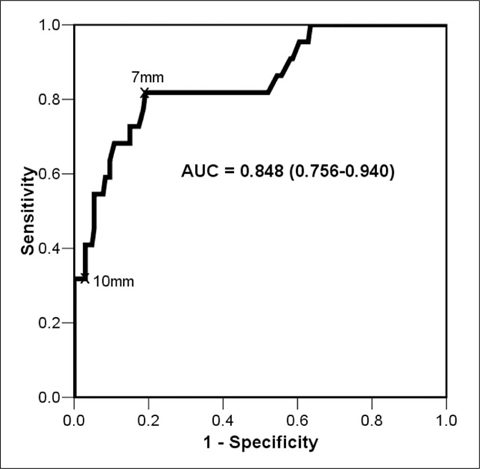
Twenty-four patients who underwent radical esophagectomy for esophageal cancer were studied. All patients had preoperative MDCT and PET-CT. The MDCT findings were compared with those of PET-CT and were correlated with the surgical records. The receiver operating characteristic (ROC) curve method was used to determine the appropriate cut-off value to distinguish benign from metastatic LNs.
RESULTS
The size of metastatic LNs (9.35+/-3.41 mm) was significantly larger than that of benign LNs (5.74+/-1.64 mm) (p<0.001). The best cut-off value was 7 mm (81.8% sensitivity, 80.8% specificity). PET-CT detected all metastatic LNs except for four in the peritumoral region. The sensitivity and specificity of metastatic LN evaluation on PET-CT were 82.6% and 99.4%, respectively. Only one LN without metastasis showed increased fluoro-2-deoxy-D-glucose uptake on PET-CT.
CONCLUSION
Size of metastatic LNs can typically be < 10 mm. For MDCT, the short diameter of 7 mm may be the optimal criterion. PET-CT is very accurate for the assessment of metastatic LNs except for those in the peritumoral region.
MeSH Terms
Figure
Reference
-
1. Roder JD, Busch R, Stein HJ, Fink U, Siewert JR. Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the oesophagus. Br J Surg. 1994; 81:410–413.2. Ellis FH Jr, Watkins E Jr, Krasna MJ, Heatley GJ, Balogh K. Staging of carcinoma of the esophagus and cardia: a comparison of different staging criteria. J Surg Oncol. 1993; 52:231–235.3. Lieberman MD, Shriver CD, Bleckner S, Burt M. Carcinoma of the esophagus: prognostic significance of histologic type. J Thorac Cardiovasc Surg. 1995; 109:130–139.4. Korst RJ, Rusch VW, Venkatraman E, Bains MS, Burt ME, Downey RJ, et al. Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg. 1998; 115:660–669.5. Choi JY, Lee KH, Shim YM, Lee KS, Kim JJ, Kim SE, et al. Improved detection of individual nodal involvement in squamous cell carcinoma of the esophagus by FDG PET. J Nucl Med. 2000; 41:808–815.6. Kato H, Kuwano H, Nakajima M, Miyazaki T, Yoshikawa M, Ojima H, et al. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer. 2002; 94:921–928.7. Kato H, Miyazaki T, Nakajima M, Takita J, Kimura H, Faried A, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer. 2005; 103:148–156.8. Halvorsen RA JR, Daffner R, Thompson WM. The esophagus. In : Godwin JD, editor. Computed tomography of the chest. Philadelphia: Lippincott;984; 247–291.9. Vilgrain V, Mompoint D, Palazzo L, Menu Y, Gayet B, Ollier P, et al. Staging of esophageal carcinoma: comparison of results with endoscopic sonography and CT. AJR Am J Roentgenol. 1990; 155:277–281.10. Goei R, Lamers RJ, Engelshove HA, Oei KT. Computed tomographic staging of esophageal carcinoma: a study on interobserver variation and correlation with pathological findings. Eur J Radiol. 1992; 15:40–44.11. Schröder W, Baldus SE, Mönig SP, Beckurts TK, Dienes HP, Hölscher AH. Lymph node staging of esophageal squamous cell carcinoma in patients with and without neoadjuvant radiochemotherapy: histomorphologic analysis. World J Surg. 2002; 26:584–587.12. Funai T, Osugi H, Higashino M, Kinoshita H. Estimation of lymph node metastasis by size in patients with intrathoracic oesophageal cancer. Br J Surg. 2000; 87:1234–1239.13. Yosiaki K, Yoshimi I, Natsumi T, Takayuki A, Fuyumi I, Toshiharu M, et al. Size analysis of lymph node metastasis in esophageal cancer: diameter distribution and assessment of accuracy of preoperative diagnosis. Esophagus. 2006; 3:189–195.14. Bruzzi JF, Munden RF, Truong MT, Marom EM, Sabloff BS, Gladish GW, et al. PET/CT of esophageal cancer: its role in clinical management. Radiographics. 2007; 27:1635–1652.15. Flanagan FL, Dehdashti F, Siegel BA, Trask DD, Sundaresan SR, Patterson GA, et al. Staging of esophageal cancer with 18F-fluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol. 1997; 168:417–424.16. Kim KM, Park SJ, Kim BT, Lee KS, Shim YM. Evaluation of lymph node metastases in squamous cell carcinoma of the esophagus with positron emission tomography. Ann Thorac Surg. 2001; 71:290–294.17. Yoon YC, Lee KS, Shim YM, Kim BT, Kim K, Kim TS. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection-prospective study. Radiology. 2003; 227:764–770.18. Yuan S, Yu Y, Chao KS, Fu Z, Yin Y, Liu T, et al. Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. J Nucl Med. 2006; 47:1255–1259.19. Bar-Shalom R, Guralnik L, Tsalic M, Leiderman M, Frenkel A, Gaitini D, et al. The additional value of PET/CT over PET in FDG imaging of oesophageal cancer. Eur J Nucl Med Mol Imaging. 2005; 32:918–924.20. Rankin SC, Taylor H, Cook GJ, Mason R. Computed tomography and positron emission tomography in the pre-operative staging of oesophageal carcinoma. Clin Radiol. 1998; 53:659–665.21. McAteer D, Wallis F, Couper G, Norton M, Welch A, Bruce D, et al. Evaluation of 18F-FDG positron emission tomography in gastric and oesophageal carcinoma. Br J Radiol. 1999; 72:525–529.22. Jager PL, Que TH, Vaalburg W, Pruim J, Elsinga P, Plukker JT. Carbon-11 choline or FDG-PET for staging of oesophageal cancer? Eur J Nucl Med. 2001; 28:1845–1849.23. Himeno S, Yasuda S, Shimada H, Tajima T, Makuuchi H. Evaluation of esophageal cancer by positron emission tomography. Jpn J Clin Oncol. 2002; 32:340–346.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reliability of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in the Nodal Staging of Colorectal Cancer Patients
- Supraclavicular Lymph Node Metastasis from Various Malignancies: Assessment with 18F-Fluorodeoxyglucose Positron Emission Tomography/CT, Contrast-Enhanced CT and Ultrasound
- Role of 18F 2-fluoro-2-deoxyglucose Positron Emission Tomography in Upper Gastrointestinal Malignancies
- F18-fluorodeoxyglucose-positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer
- The Use of PET in Esophageal Cancer