J Korean Rheum Assoc.
2007 Sep;14(3):219-226. 10.4078/jkra.2007.14.3.219.
Characterization of FSC(high) Memory B Cells from Patients with Active Systemic Lupus Erythematosus
- Affiliations
-
- 1The Rheumatism Research Center (RhRC), Catholic Research Institute of Medical Science, The Catholic University of Korea, Seoul, Korea. rheuma@catholic.ac.kr
- 2and Department of Internal Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2202170
- DOI: http://doi.org/10.4078/jkra.2007.14.3.219
Abstract
OBJECTIVE
To determine phenotypic and functional characteristics of memory B cells in patients with systemic lupus erythematosus (SLE).
METHODS
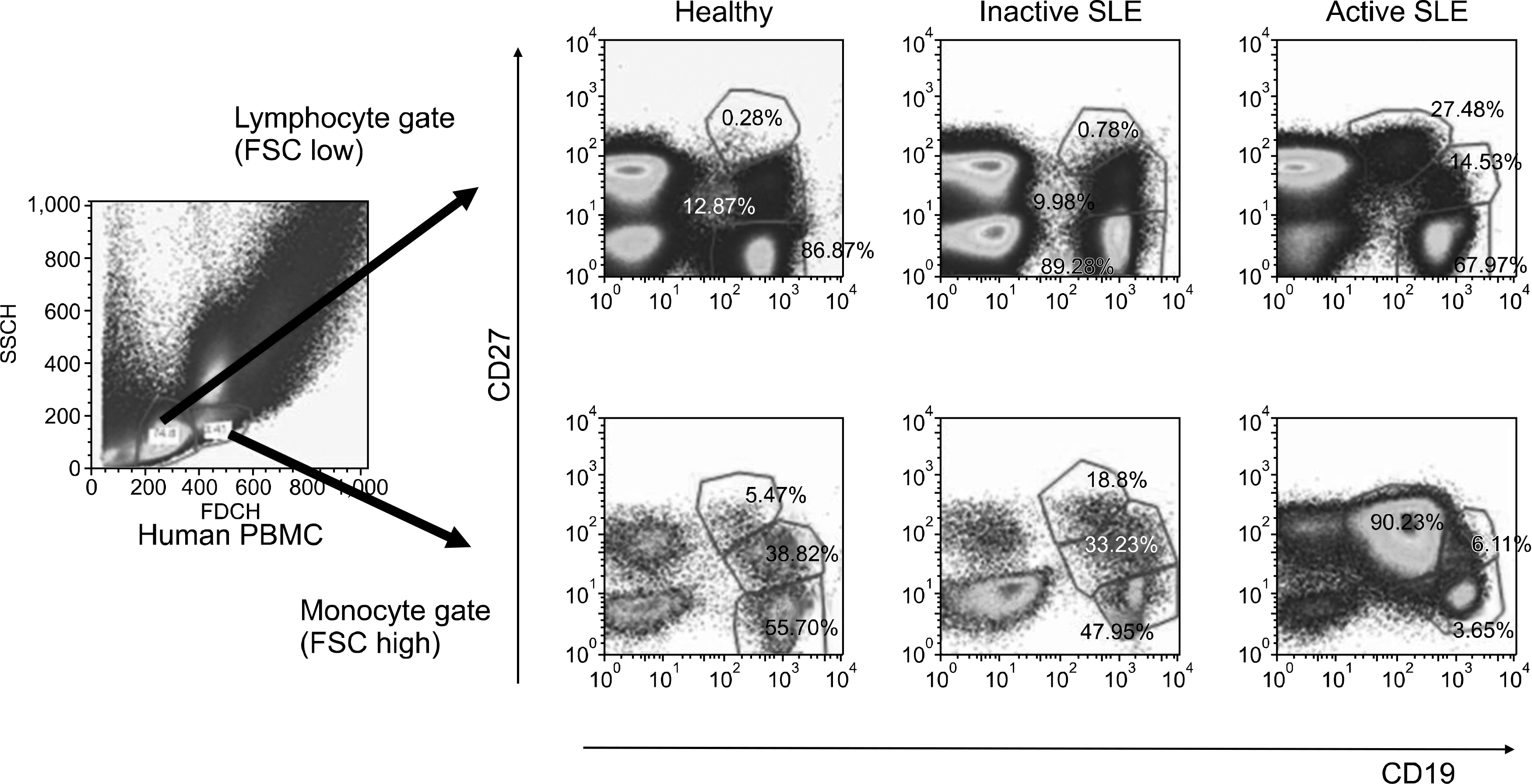
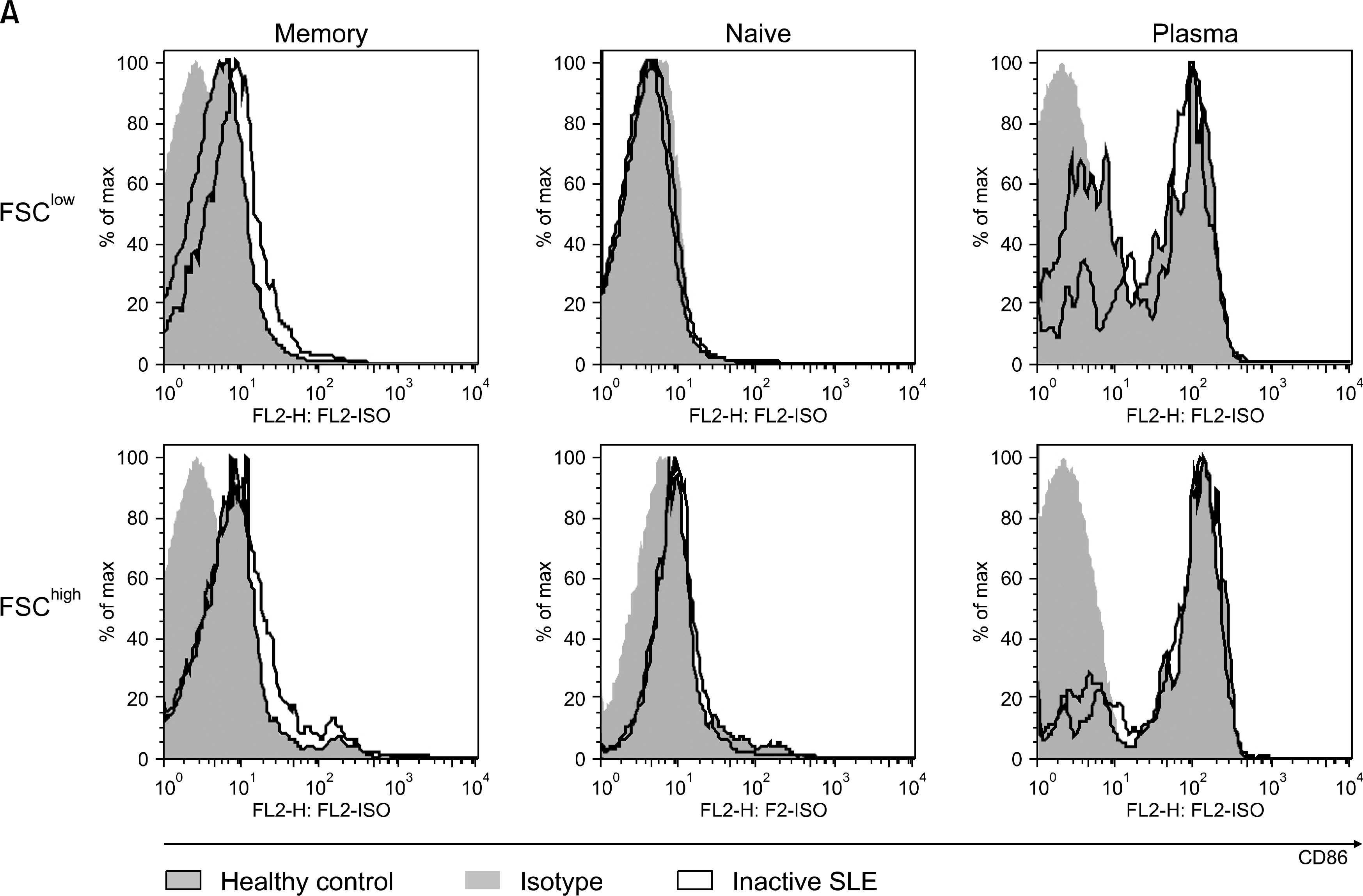
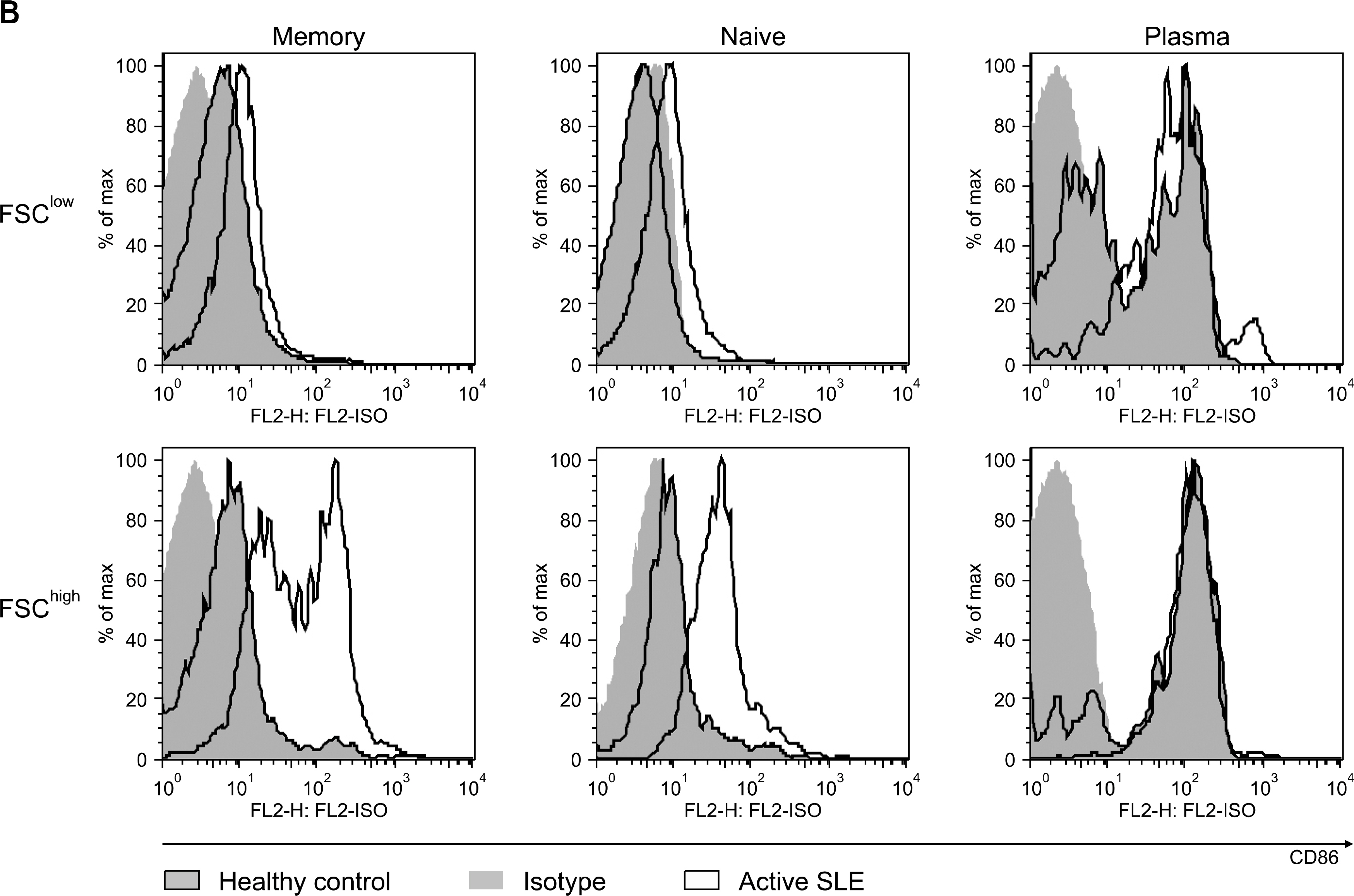
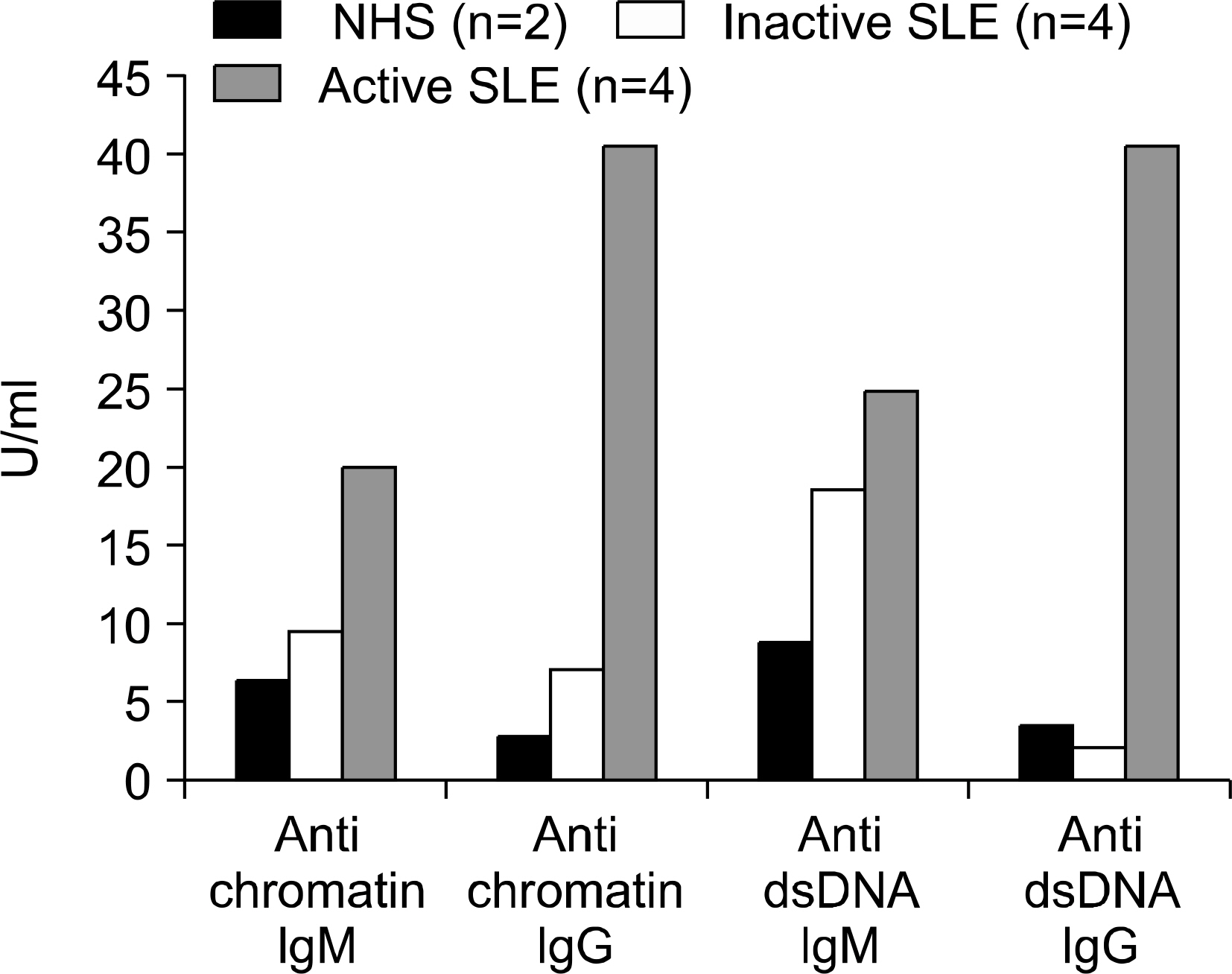
The percentage of memory B cell subsets in peripheral blood mononuclear cells (PBMC) from normal control (n=11), inactive (n=15) and active (n=10) SLE patients was determined by Fluorescence Activated Cell Sorter (FACS). In addition, the activation status of memory B cells was measured by the surface expression of CD86 (B7-2). The production of antibodies to chromatin and dsDNA (IgG and IgM type) by isolated memory B cell subsets was examined by enzyme-linked immunosorbent assay (ELISA).
RESULTS
In this study, we analyzed 2 subtypes of memory B cells: FSC (Forward Side Scatter)(low) and FSC(high) memory B cell. The percentage of both subtypes from active and inactive SLE patients was significantly reduced compared to that of normal controls (p<0.01). In addition, the expression of activation markers, CD86 on FSC(high) memory B cells from active SLE patients was higher than those of inactive SLE patients and normal controls (p=0.014). Upon stimulation with CpG and IL-15 in vitro for 8 days, isolated FSC(high) memory B cells from active SLE patients revealed augmented production of autoantibodies to chromatin and dsDNA.
CONCLUSION
Our results suggest that abnormally activated FSC(high) memory B cells from active SLE patients might be involved in spontaneous production of autoantibodies and induce transition from inactive to active phase of the patients.
MeSH Terms
Figure
Reference
-
1). Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nature Immunol. 2001. 2:764–6.
Article2). Thomas HW., Holger F., Joachim R., Kalden . Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur J Immunol. 1992. 22:1718–28.3). Gregory CI., Robert LS., Michael Z., Ivaylo I., Ryoki K., Cosima Z, et al. Forced usage of positively charged amino acids in immunoglobulin CDR-H3 impairs B cell development and antibody production. J Exp Med. 2006. 203:1567–78.4). Wellman U., Letz M., Hermann M., Angermuller S., Kalden JR., Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci USA. 2005. 102:9258–63.5). Mark JS., Ann HA., David SP., Martin GW. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. PNAS. 1987. 84:9150–4.6). Dan E., Malka H., Francois T., Laurent J., Jean-Francois B. The VH gene sequences of anti-DNA antibodies in two different strains of lupus prone mice are highly related. Eur J Immunol. 1989. 19:1241–6.7). Marion TN., Tillman DM., Jou NT. Interclinal and intraclonal diversity among anti-DNA antibodies from (NZBxNZW) F1 mouse. J Immunol. 1990. 145:2322–32.8). Louise J., Michael G. Antigen-Specific Memory B cell development. Annu Rev Immunol. 2005. 23:487–513.9). Neal NI., Ann HL., Prasanth V., Kevin LO., Klaus R., Laurie HG. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nature immunology. 2003. 4:321–9.10). Bernasconi NL., Traggiai E., Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002. 298:291–4.
Article11). Wirths S., Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol. 2005. 35:3433–41.12). Jacobi AM., Odendahl M., Reiter K., Bruns A., Burmester GR., Radbrunch A, et al. Correlation between circulating CD27high plasma cells and disease activity in patients activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003. 48:1332–42.13). Sato S., Fujimoto M., Hasegawa M., Takehara K. Altered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cells. Arthritis Rheum. 2004. 50:1918–27.
Article14). Bombardier D., Cladman DD., Urowith MB., Chang CH., and the Committee on Prognosis Studies in SLE. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992. 35:630–40.
Article15). Odendahl M., Jacobi A., Hansen A., Feist E., Hiepe F., Burmester GR, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000. 165:5970–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Transverse Myelitis as a First Manifestation of Systemic Lupus Erythematosus
- A Case of Lupus Enteritis That Developed during the Treatment of Systemic Lupus Erythematosus
- A Case Of Systemic Lupus Erythematosus Associated With Hyperthyroidism And Severe Retinopathy
- Multiple Dermatofibromas in a woman with Systemic Lupus Erythematosus
- A Case of Active Systemic Lupus Erythematosus Successful Treated with Plasmapheresis