J Korean Med Assoc.
2015 Sep;58(9):801-808. 10.5124/jkma.2015.58.9.801.
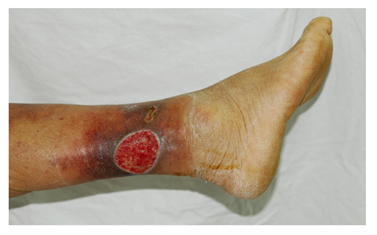
Radiation ulcers and other chronic wounds
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Korea University Medical Center, Korea University College of Medicine, Ansan, Korea. deokwookim@gmail.com
- KMID: 2195092
- DOI: http://doi.org/10.5124/jkma.2015.58.9.801
Abstract
- Radiation ulcers are wounds caused by acute or chronic effects of ionizing radiation. The injury may involve the skin, underlying soft tissue, and even deeper into bones. Radiation is used alone or in combination with surgery and chemotherapy. Although it is useful to affect tumor death, it also exerts a deleterious effect on surrounding normal tissues. These effects are either acute or can manifest months or years after the treatment. The chronic wounds are a result of impaired wound healing. This impairment leads to fibrosis, nonhealing ulcers, lymphedema and radionecrosis amongst others.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Chronic wound
Jong Won Rhie
J Korean Med Assoc. 2015;58(9):784-785. doi: 10.5124/jkma.2015.58.9.784.
Reference
-
1. Maddocks-Jennings W, Wilkinson JM, Shillington D. Novel approaches to radiotherapy-induced skin reactions: a literature review. Complement Ther Clin Pract. 2005; 11:224–231.
Article2. Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freed-man GM, Asrari F, Pilepich MV, Gagnon JD, Wong G. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000; 48:1307–1310.
Article3. Sciubba JJ, Goldenberg D. Oral complications of radiotherapy. Lancet Oncol. 2006; 7:175–183.
Article4. National Cancer Institute. Common terminology criteria for adverse events v.3.0 (CTCAE) [Internet]. Bethesda: National Cancer Institute;2010. cited 2015 Sep 9. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.5. Simonen P, Hamilton C, Ferguson S, Ostwald P, O'Brien M, O'Brien P, Back M, Denham J. Do inflammatory processes contribute to radiation induced erythema observed in the skin of humans? Radiother Oncol. 1998; 46:73–82.
Article6. Schmuth M, Sztankay A, Weinlich G, Linder DM, Wimmer MA, Fritsch PO, Fritsch E. Permeability barrier function of skin exposed to ionizing radiation. Arch Dermatol. 2001; 137:1019–1023.7. Kupper TS. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Invest Dermatol. 1990; 94:6 Suppl. 146S–150S.8. Mendelsohn FA, Divino CM, Reis ED, Kerstein MD. Wound care after radiation therapy. Adv Skin Wound Care. 2002; 15:216–224.
Article9. Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995; 31:1171–1185.
Article10. Peters LJ, Ang KK, Thames HD Jr. Accelerated fractionation in the radiation treatment of head and neck cancer: a critical comparison of different strategies. Acta Oncol. 1988; 27:185–194.
Article11. Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001; 61:223–231.
Article12. Denham JW, Hauer-Jensen M. The radiotherapeutic injury: a complex 'wound'. Radiother Oncol. 2002; 63:129–145.13. Malkinson FD, Keane JT. Radiobiology of the skin: review of some effects on epidermis and hair. J Invest Dermatol. 1981; 77:133–138.
Article14. Lyons A, Ghazali N. Osteoradionecrosis of the jaws: current understanding of its pathophysiology and treatment. Br J Oral Maxillofac Surg. 2008; 46:653–660.
Article15. Delanian S, Porcher R, Balla-Mekias S, Lefaix JL. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003; 21:2545–2550.
Article16. Hutcheson KA, Lewin JS. Functional outcomes after chemo-radiotherapy of laryngeal and pharyngeal cancers. Curr Oncol Rep. 2012; 14:158–165.
Article17. Delanian S, Depondt J, Lefaix JL. Major healing of refractory mandible osteoradionecrosis after treatment combining pentoxifylline and tocopherol: a phase II trial. Head Neck. 2005; 27:114–123.
Article18. Chang EI, Leon P, Hoffman WY, Schmidt BL. Quality of life for patients requiring surgical resection and reconstruction for mandibular osteoradionecrosis: 10-year experience at the University of California San Francisco. Head Neck. 2012; 34:207–212.
Article19. Baumann DP, Yu P, Hanasono MM, Skoracki RJ. Free flap reconstruction of osteoradionecrosis of the mandible: a 10-year review and defect classification. Head Neck. 2011; 33:800–807.
Article20. McGuirt WF. Laryngeal radionecrosis versus recurrent cancer. Otolaryngol Clin North Am. 1997; 30:243–250.
Article21. Galecki J, Hicer-Grzenkowicz J, Grudzien-Kowalska M, Michalska T, Zalucki W. Radiation-induced brachial plexopathy and hypofractionated regimens in adjuvant irradiation of patients with breast cancer: a review. Acta Oncol. 2006; 45:280–284.
Article22. Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol. 2010; 177:3202–3214.
Article23. Bruns F, Micke O, Bremer M. Current status of selenium and other treatments for secondary lymphedema. J Support Oncol. 2003; 1:121–130.24. Mucke T, Rau A, Weitz J, Ljubic A, Rohleder N, Wolff KD, Mitchell DA, Kesting MR. Influence of irradiation and oncologic surgery on head and neck microsurgical reconstructions. Oral Oncol. 2012; 48:367–371.
Article25. Halle M, Bodin I, Tornvall P, Wickman M, Farnebo F, Arnander C. Timing of radiotherapy in head and neck free flap reconstruction: a study of postoperative complications. J Plast Reconstr Aesthet Surg. 2009; 62:889–895.
Article26. Chandan K, Sen R. Chronic wounds. In : Neligan PC, editor. Plastic surgery. 3rd ed. New York: Elsevier Saunders;2013. p. 253–260.27. Grover-Paez F, Garcia-Benavides L, Cardona-Munoz EG, Ramos-Becerra CG, Garcia-Corral JR, Medina-Garcia CE, Alanis-Sanchez GA, Totsuka-Sutto SE. Successful manage-ment of a complicated ulcer caused by autoimmune vasculitis, with sildenafil citrate in a patient with primary antiphospholipid syndrome and transient steroid-induced diabetes mellitus: a case report and literature review. J Diabetes Mellitus. 2014; 4:189–193.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Coccygeal Radiation-Induced Ulcer Using Injectable Acellular Dermal Matrix Combined with Negative Pressure Wound Therapy: A Case Report
- The Clinical Uses of Collagen-Based Matrices in the Treatment of Chronic Wounds
- Abdominoplasty Combined with Wide Excision in Marjolin's Ulcer: Report of 2 Cases
- Radiation therapy in chronic hidradenitis suppurativa: case report
- A Case of Leg Ulcer in Systemic Scleroderma Treated with Pinch Grafts