J Korean Med Assoc.
2015 May;58(5):385-397. 10.5124/jkma.2015.58.5.385.
The Korean guideline for hepatocellular carcinoma surveillance
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 2Institute for Preventive Medicine, Korea University College of Medicine, Seoul, Korea.
- 3Department of Radiology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Soonchunhyang University College of Medicine, Cheonan, Korea. khskhs@sch.ac.kr
- 5Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 6Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 7Department of Family Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 8Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 9Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea.
- 10Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 11Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea.
- 12Chamsarang Medical Clinic, Seoul, Korea.
- 13Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 14Department of Occupational and Environmental Medicine, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 15Department of Family Medicine, Seoul National University Hospital, Seoul, Korea.
- 16Department of Radiology, National Cancer Center, Goyang, Korea.
- 17Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- 18National Cancer Control Institute, National Cancer Center, Goyang, Korea.
- 19Department of Family Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 20Department of Preventive Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2195035
- DOI: http://doi.org/10.5124/jkma.2015.58.5.385
Abstract
- Hepatocellular carcinoma (HCC) is one of the major cancers with a high incidence and mortality in Korea. A Korean multidisciplinary collaborative committee consisting of hepatologists, radiologists, epidemiologists and family medicine doctors systematically reviewed clinical practice guidelines in the world and literatures. The level of evidence for each recommendation was assessed and discussed to reach a consensus. Meta-analysis was also conducted to evaluate the grade of recommendation for the five key questions. Several randomized controlled studies and cohort studies showed a survival gain associated with surveillance for those at risk of developing HCC. The target populations for HCC surveillance were identified as hepatitis B virus or hepatitis C virus carriers and cirrhotic patients, since numerous studies revealed that these patients have significantly higher risk of HCC compared with non-infected or non-cirrhotic controls. Individual surveillance strategy according to treatment history or degree of fibrosis in patients with viral hepatitis remains to be settled. Based on several cohort and randomized studies, a surveillance interval of six months was recommend. The starting age of surveillance was determined as 40 years from the epidemiologic data. Although ultrasonography (US) is the mainstay for detection of HCC, its sensitivity is not fully accepted. Measurement of serum alpha-fetoprotein can complement US examination, increasing the sensitivity of HCC detection. The recommendation for HCC surveillance is that those with hepatitis B virus (or hepatitis C virus) infection or cirrhosis should have liver US and serum alpha-fetoprotein measurement every six months from 40 years of age or at the time of diagnosis of cirrhosis.
MeSH Terms
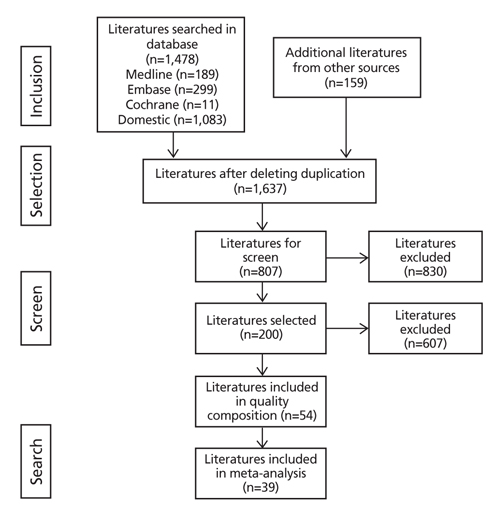
Figure
Cited by 3 articles
-
Quality management of medical imaging for public health screening
Woo Kyoung Jeong, Eun Hye Lee, Seung Eun Jung
J Korean Med Assoc. 2015;58(12):1125-1131. doi: 10.5124/jkma.2015.58.12.1125.2018 Korean Liver Cancer Association–National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma
,
Korean J Radiol. 2019;20(7):1042-1113. doi: 10.3348/kjr.2019.0140.Surveillance Rate and its Impact on Survival of Hepatocellular Carcinoma Patients in South Korea: A Cohort Study
Sanghyuk Im, Eun Sun Jang, Ju Hyun Lee, Chung Seop Lee, Beom Hee Kim, Jung Wha Chung, Jin-Wook Kim, Sook-Hyang Jeong
Cancer Res Treat. 2019;51(4):1357-1369. doi: 10.4143/crt.2018.430.
Reference
-
1. Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, Shiina S, Cheng AL, Jia JD, Obi S, Han KH, Jafri W, Chow P, Lim SG, Chawla YK, Budihusodo U, Gani RA, Lesmana CR, Putranto TA, Liaw YF, Sarin SK. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010; 4:439–474.
Article2. Singapore Ministry of Health. Cancer screening: clinical practice guidelines. Singapore: Ministry of Health;2010.3. New Zealand Ministry of Health; New Zealand Guidelines Group. Suspected cancer in primary care: guidelines for investigation, referral and reducing ethnic disparities. Wellington: New Zealand Guidelines Group;2009.4. National Institute for Health and Care Excellence. Hepatitis B (chronic): diagnosis and management of chronic hepatitis B in children, young people and adults [Internet]. London: National Institute for Health and Care Excellence;2013. cited 2015 Apr 27. Available from: https://www.nice.org.uk/guidance/cg165.5. Kokudo N, Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol. 2009; 44:Suppl 19. 119–121.
Article6. Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013; 58:593–608.
Article7. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.
Article8. Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009; 15:391–423.9. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004; 130:417–422.
Article10. Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, Zhu YR. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003; 10:204–209.
Article11. Aghoram R, Cai P, Dickinson JA. Alpha-foetoprotein and/or liver ultrasonography for screening of hepatocellular carcinoma in patients with chronic hepatitis B. Cochrane Database Syst Rev. 2012; 9:CD002799.
Article12. Trevisani F, Cantarini MC, Labate AM, De Notariis S, Rapaccini G, Farinati F, Del Poggio P, Di Nolfo MA, Benvegnu L, Zoli M, Borzio F, Bernardi M; Italian Liver Cancer (ITALICA) group. Surveillance for hepatocellular carcinoma in elderly Italian patients with cirrhosis: effects on cancer staging and patient survival. Am J Gastroenterol. 2004; 99:1470–1476.
Article13. Yoo KY, Heon-Kim , Lee MS, Park BJ, Ahn YO, Lee HS, Kim CY, Park TS. A reconstructed cohort study on the hepatitis B virus infection as a risk factor of liver cancer in Korea. J Korean Med Sci. 1991; 6:319–324.
Article14. El-Serag HB, Kramer JR, Chen GJ, Duan Z, Richardson PA, Davila JA. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut. 2011; 60:992–997.
Article15. Huh K, Lee JK, Choi SY, Hong SI, Lee DS. A study on the prevalence of HBsAg and Anti-HCV in patients with hepatocellular carcinoma: comparative study with healthy blood donors. Korean J Clin Pathol. 1998; 18:458–463.16. Tsai JF, Jeng JE, Ho MS, Chang WY, Hsieh MY, Lin ZY, Tsai JH. Additive effect modification of hepatitis B surface antigen and e antigen on the development of hepatocellular carcinoma. Br J Cancer. 1996; 73:1498–1502.
Article17. Lee HS, Lee JH, Choi MS, Kim CY. Comparison of the incidence of hepatocellular carcinoma in HBV-and HCV-associated liver cirrhosis: a prospective study. Korean J Hepatol. 1996; 2:21–28.18. Jee SH, Ohrr H, Sull JW, Samet JM. Cigarette smoking, alcohol drinking, hepatitis B, and risk for hepatocellular carcinoma in Korea. J Natl Cancer Inst. 2004; 96:1851–1856.
Article19. Evans AA, Chen G, Ross EA, Shen FM, Lin WY, London WT. Eight-year follow-up of the 90,000-person Haimen City cohort. I. Hepatocellular carcinoma mortality, risk factors, and gender differences. Cancer Epidemiol Biomarkers Prev. 2002; 11:369–376.20. Sun CA, Wu DM, Lin CC, Lu SN, You SL, Wang LY, Wu MH, Chen CJ. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. Am J Epidemiol. 2003; 157:674–682.
Article21. Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993; 328:1797–1801.
Article22. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J; Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004; 351:1521–1531.
Article23. Moon C, Jung KS, Kim do Y, Baatarkhuu O, Park JY, Kim BK, Kim SU, Ahn SH, Han KH. Lower incidence of hepatocellular carcinoma and cirrhosis in hepatitis C patients with sustained virological response by pegylated interferon and ribavirin. Dig Dis Sci. 2015; 60:573–581.
Article24. Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, Nishiguchi S, Kuroki T, Imazeki F, Yokosuka O, Kinoyama S, Yamada G, Omata M. Interferon therapy reduces the risk for hepato-cellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999; 131:174–181.
Article25. Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, Lobello S, Farinati F, Naccarato R. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999; 85:2132–2137.
Article26. Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Pis-caglia F, Gramantieri L, Zanetti M, Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001; 48:251–259.
Article27. Pateron D, Ganne N, Trinchet JC, Aurousseau MH, Mal F, Meicler C, Coderc E, Reboullet P, Beaugrand M. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994; 20:65–71.
Article28. Han KH, Kim do Y, Park JY, Ahn SH, Kim J, Kim SU, Kim JK, Lee KS, Chon CY. Survival of hepatocellular carcinoma patients may be improved in surveillance interval not more than 6 months compared with more than 6 months: a 15-year prospective study. J Clin Gastroenterol. 2013; 47:538–544.
Article29. Santagostino E, Colombo M, Rivi M, Rumi MG, Rocino A, Linari S, Mannucci PM; Study Group of the Association of Italian Hemophilia Centers. A 6-month versus a 12-month surveillance for hepatocellular carcinoma in 559 hemophiliacs infected with the hepatitis C virus. Blood. 2003; 102:78–82.
Article30. Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, Di Nolfo MA, Benvegnu L, Farinati F, Zoli M, Giannini EG, Borzio F, Caturelli E, Chiaramonte M, Bernardi M; Italian Liver Cancer (ITA.LI.CA) Group. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010; 53:291–297.
Article31. Wang JH, Chang KC, Kee KM, Chen PF, Yen YH, Tseng PL, Kuo YH, Tsai MC, Hung CH, Chen CH, Tai WC, Tsai LS, Chen SC, Lin SC, Lu SN. Hepatocellular carcinoma surveillance at 4- vs. 12-month intervals for patients with chronic viral hepatitis: a randomized study in community. Am J Gastroenterol. 2013; 108:416–424.
Article32. Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009; 30:37–47.
Article33. National Cancer Center. Korean Liver Cancer Study Group. 2014 Practice guidelines for the management of hepatocellular carcinoma. Goyang: National Cancer Center;2014.34. Kudo M. Real practice of hepatocellular carcinoma in Japan: conclusions of the Japan Society of Hepatology 2009 Kobe Congress. Oncology. 2010; 78:Suppl 1. 180–188.
Article35. Cottone M, Turri M, Caltagirone M, Parisi P, Orlando A, Fiorentino G, Virdone R, Fusco G, Grasso R, Simonetti RG. Screening for hepatocellular carcinoma in patients with child's A cirrhosis: an 8-year prospective study by ultrasound and alphafetoprotein. J Hepatol. 1994; 21:1029–1034.
Article36. Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004; 126:1005–1014.
Article37. Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006; 43:1303–1310.
Article38. Tradati F, Colombo M, Mannucci PM, Rumi MG, De Fazio C, Gamba G, Ciavarella N, Rocino A, Morfini M, Scaraggi A, Taioli E. A prospective multicenter study of hepatocellular carcinoma in italian hemophiliacs with chronic hepatitis C. The Study Group of the Association of Italian Hemophilia Centers. Blood. 1998; 91:1173–1177.39. Wong GL, Chan HL, Tse YK, Chan HY, Tse CH, Lo AO, Wong VW. On-treatment alpha-fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology. 2014; 59:986–995.
Article40. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009; 49:1017–1044.
Article41. Committee to assess health risks from exposure to low levels of ionizing radiation, national research council. Health risks from exposure to low levels of ionizing radiation: BEIR VII-Phase 2. The national academies press;2006.42. Aleman S, Rahbin N, Weiland O, Davidsdottir L, Hedenstierna M, Rose N, Verbaan H, Stal P, Carlsson T, Norrgren H, Ekbom A, Granath F, Hultcrantz R. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis. 2013; 57:230–236.
Article43. Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M, Saida Y, Takayama T, Yamaoka Y. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008; 38:37–51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surveillance of hepatocellular carcinoma: is only ultrasound enough?
- What should be done to reduce the discrepancy between guidelines and real-life practice for hepatocellular carcinoma in Korea?
- Surveillance for hepatocellular carcinoma: It is time to move forward
- 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma
- Hepatocellular carcinoma surveillance: Eastern and Western perspectives