J Korean Med Assoc.
2014 Jan;57(1):49-59. 10.5124/jkma.2014.57.1.49.
An introduction to systematic review
- Affiliations
-
- 1Department of Preventive Medicine, Korea University College of Medicine, Seoul, Korea. ahnhs@korea.ac.kr
- KMID: 2194834
- DOI: http://doi.org/10.5124/jkma.2014.57.1.49
Abstract
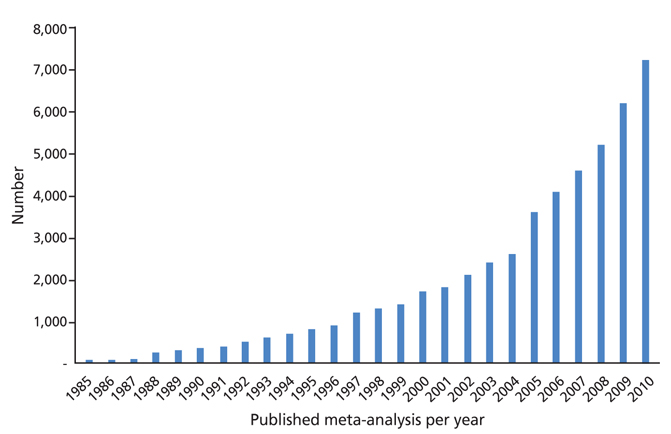
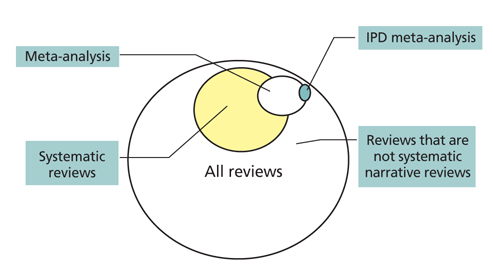
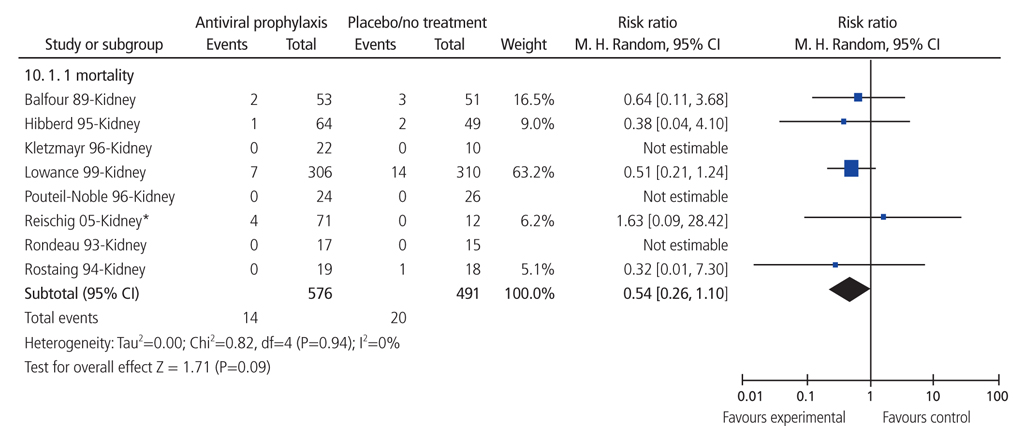
- Systematic review had become one of the important research area in medicine. Systematic review can be demonstrating benefit or harm of an intervention when results of individual studies are inconclusive. While narrative reviews can often include an element of selection bias, systematic reviews typically involve a comprehensive plan and search strategy with the goal of reducing bias by identifying, appraising, and synthesizing all relevant studies on a particular topic and investigation of heterogeneity among included studies. Systematic reviews typically include a meta-analysis component which involves using statistical techniques to synthesize the data from several studies into a single quantitative estimate or summary effect size. Systematic review overcomes the limitation of small sample sizes by pooling results from a number of individual studies to generate a single best estimate. Although systematic reviews are published in academic forums, the Cochrane Collaboration is a widely recognized international and not-for-profit organization that promotes, supports, and disseminates systematic reviews and meta-analyses on the efficacy of interventions in the health care field. Systematic review has become a popular and powerful tool. If rigorously conducted, it is essential for evidence-based decision making in clinical practice as well as on the health policy level.
Keyword
MeSH Terms
Figure
Reference
-
1. Report on certain enteric fever inoculation statistics. Br Med J. 1904; 2:1243–1246.2. Glass GV. Primary, secondary and meta-analysis of research. Educ Res. 1976; 5:3–8.
Article3. Mulrow CD. The medical review article: state of the science. Ann Intern Med. 1987; 106:485–488.
Article4. Cochrane AL. Effectiveness and efficiency: random reflections on health services. London: Royal Society of Medicine Press;1999.5. Cochrane AL. 1931-1971: a critical review, with particular reference to the medical profession. In : Teeling-Smith G, Wells NE, editors. Office of Health Economics. Medicines for the year 2000. London: Office of Health Economics;1979. p. 1–11.6. Bero L, Rennie D. The Cochrane Collaboration. Preparing, maintaining, and disseminating systematic reviews of the effects of health care. JAMA. 1995; 274:1935–1938.
Article7. Heejeng Son. Assessing methodology quality of Korean systematic review using AMSTAR. Seoul: Korea university;2012.8. McAlister FA, Clark HD, van Walraven C, Straus SE, Law-son FM, Moher D, Mulrow CD. The medical review article revisited: has the science improved? Ann Intern Med. 1999; 131:947–951.
Article9. Bravata DM, Olkin I. Simple pooling versus combining in meta-analysis. Eval Health Prof. 2001; 24:218–230.
Article10. Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009; 26:91–108.
Article11. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. Cochrane Bias Methods Group. Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article12. Petitti DB. Meta-analysis, decision analysis, and cost-effectiveness analysis: methods for quantitative synthesis in medicine. New York: Oxford University Press;1999.13. Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. 1st ed. Chichester: Wiley;2000.14. Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta-analysis in context. . London: BMJ Books;2001.15. Glasziou P, Irwig L, Bain C, Colditz G. Systematic reviews in health care: a practical guide. Cambridge: Cambridge University Press;2001.16. Stangl DK, Berry DA. Meta-analysis in medicine and health policy. Basel: Marcel Dekker;2000.17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.
Article18. Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994; 271:698–702.
Article19. Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA. 1990; 263:1385–1389.
Article20. Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008; 358:252–260.
Article21. Yusuf S, Teo K, Woods K. Intravenous magnesium in acute myocardial infarction. An effective, safe, simple, and inexpensive intervention. Circulation. 1993; 87:2043–2046.
Article22. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarctions. Lancet. 1995; 345:669–685.23. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–269.
Article24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009; 6:e1000100.
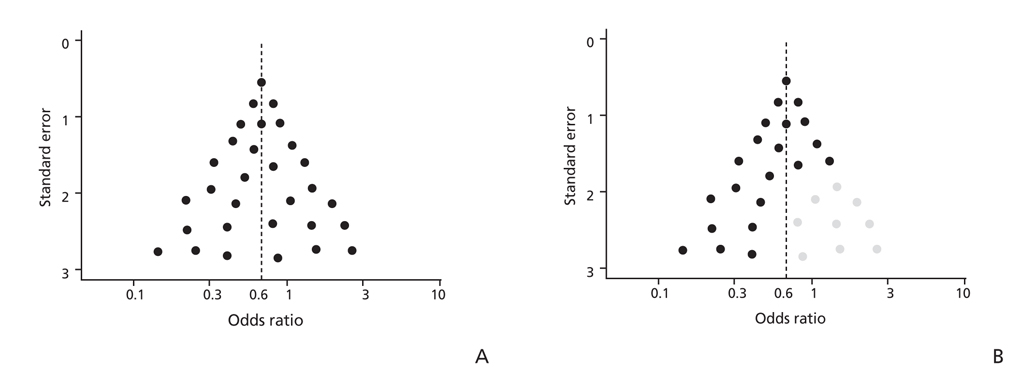
Article25. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rucker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011; 343:d4002.
Article26. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, Porter AC, Tugwell P, Moher D, Bouter LM. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007; 7:10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Introduction of the Systematic Review and Meta-Analysis
- Introduction to systematic review and meta-analysis
- Systematic review for new health technology assessment
- Current status of systematic review studies on patient-reported outcome measures published in Korean journals
- Interventions on Well-being, Occupational Health, and Aging of Healthcare Workers: A Scoping Review of Systematic Reviews