J Korean Assoc Oral Maxillofac Surg.
2012 Apr;38(2):90-95. 10.5125/jkaoms.2012.38.2.90.
Prophylactic antibiotics in intra-oral bone grafting procedures: a prospective, randomized, double-blind clinical trial
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Seoul National University, Seoul, Korea. leejongh@snu.ac.kr
- KMID: 2189699
- DOI: http://doi.org/10.5125/jkaoms.2012.38.2.90
Abstract
OBJECTIVES
This study was conducted in order to assess the efficacy of 1st generation cephalosporin as use as a single-dose preoperative prophylactic antibiotic for surgical wound infections resulting from intra-oral bone grafting procedures.
MATERIALS AND METHODS
A total of 23 patients who were to undergo intra-oral bone graft procedures participated in this study. After randomization, 2 grams of 1st generation cephalosporin was orally administered to both the experimental and placebo groups one hour prior to surgery in a double-blind fashion. Post-operatively, the experimental group (12 patients) was orally administered placebo three times a day for three days. The control group (11 patients) was orally administered 1st generation cephalosporin three times a day for three days. The postoperative course was observed for one month including the clinical parameters associated with infection.
RESULTS
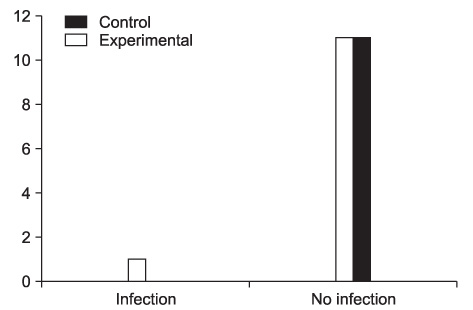
Postoperative infections were noted in 1 out of 11 patients in the experimental group. No infections occurred in the control group.
CONCLUSION
There was no significant difference in the incidence of postoperative infections between the two groups. Two grams of 1st generation cephalosporin administered orally one hour before surgery served as an effective prophylactic antibiotics therapy for intra-oral bone graft surgery
MeSH Terms
Figure
Cited by 1 articles
-
Overview of Antibiotic Use in Korea
Baek-Nam Kim
Infect Chemother. 2012;44(4):250-262. doi: 10.3947/ic.2012.44.4.250.
Reference
-
1. Kang SH, Choi YS, Byun IY, Kim MK. Antibiotic prophylaxis in the operation of the closed mandibular fractures and the efficacy of postoperative antibiotics. J Korean Assoc Oral Maxillofac Surg. 2009. 35:31–34.2. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999. 20:250–278.3. Ko JK, Cho YK, Yang HJ, Park CW, Park JS, Jun JK, et al. A prospective multicenter randomized study on prophylactic antibiotics use in cesarean section performed at tertiary center. Korean J Obstet Gynecol. 2010. 53:227–234.
Article4. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee. American Heart Association Council on Cardiovascular Disease in the Young. American Heart Association Council on Clinical Cardiology. American Heart Association Council on Cardiovascular Surgery and Anesthesia. Quality of Care and Outcomes Research Interdisciplinary Working Group. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007. 116:1736–1754.5. Tong DC, Rothwell BR. Antibiotic prophylaxis in dentistry: a review and practice recommendations. J Am Dent Assoc. 2000. 131:366–374.
Article6. Alrashdan M, Choung HU, Pang KM, Park JC, Kim SM, Kim MJ, et al. Literature review of antibiotics prescription in general dental and oral-maxillofacial surgical practice. J Korean Assoc Oral Maxillofac Surg. 2009. 35:164–169.7. Monaco G, Staffolani C, Gatto MR, Checchi L. Antibiotic therapy in impacted third molar surgery. Eur J Oral Sci. 1999. 107:437–441.
Article8. Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992. 326:281–286.
Article9. Poeschl PW, Eckel D, Poeschl E. Postoperative prophylactic antibiotic treatment in third molar surgery--a necessity? J Oral Maxillofac Surg. 2004. 62:3–8.
Article10. Monaco G, Staffolani C, Gatto MR, Checchi L. Antibiotic therapy in impacted third molar surgery. Eur J Oral Sci. 1999. 107:437–441.
Article11. Lindeboom JA, Frenken JW, Tuk JG, Kroon FH. A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone-grafting procedures: preoperative single-dose penicillin versus preoperative single-dose clindamycin. Int J Oral Maxillofac Surg. 2006. 35:433–436.
Article12. Lindeboom JA, van den Akker HP. A prospective placebo-controlled double-blind trial of antibiotic prophylaxis in intraoral bone grafting procedures: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 96:669–672.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Evaluation of Antibiotics Prophylaxis Against Infection in Clean Orthopaedic Surgery
- Efficacy of Trimetazidine Dihydrochloride for Relieving Chronic Tinnitus: A Randomized Double-Blind Study
- The Efficacy of Postoperative Prophylactic Antibiotics in Orthognathic Surgery: A Prospective Study in Le Fort I Osteotomy and Bilateral Intraoral Vertical Ramus Osteotomy
- Celecoxib Versus Diclofenac Plus Omeprazole in High-risk Arthritis Patients:Results of a Randomized Double-blind Trial
- Comparison of ondansetron and granisetron for antiemetic prophylaxis in maxillofacial surgery patients receiving general anesthesia: a prospective, randomised, and double blind study