Allergy Asthma Immunol Res.
2014 Sep;6(5):428-433. 10.4168/aair.2014.6.5.428.
Serial Changes in Serum Eosinophil-associated Mediators between Atopic and Non-atopic Children after Mycoplasma pneumoniae pneumonia
- Affiliations
-
- 1Department of Pediatrics, Hanyang University College of Medicine, Seoul, Korea. jaewonoh@hanyang.ac.kr
- KMID: 2181079
- DOI: http://doi.org/10.4168/aair.2014.6.5.428
Abstract
- PURPOSE
Mycoplasma pneumoniae pneumonia (MP) is associated with the exacerbation, timing, and onset of asthma. The goal of this study was to elucidate the impact of MP on eosinophil-related hyper-reactive amplification in atopic children.
METHODS
We studied 48 patients with MP (26 atopic, 22 non-atopic), between 3 and 12 years of age. Serial changes in blood eosinophil counts, serum interleukin-5 (IL-5), and serum eosinophil cationic protein (ECP) levels were measured in atopic and non-atopic children with MP upon admission, recovery, and at 2 months post-recovery. Serum IL-5 and ECP levels were measured by enzyme-linked immunosorbent assays; eosinophil counts were measured using an autoanalyzer.
RESULTS
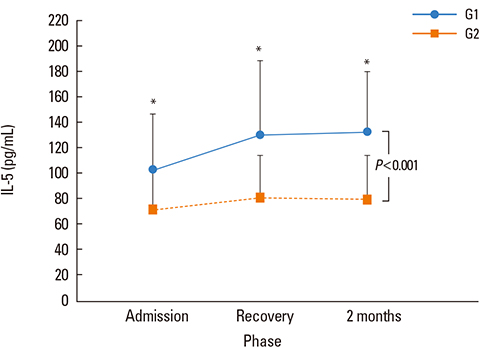
Serial changes in serum IL-5, ECP, and total eosinophil counts were significantly higher in atopic patients, relative to non-atopic controls (P< or =0.001). Serum IL-5 and ECP levels were significantly higher in atopic patients at all three time points tested, while eosinophil counts were higher in the clinical recovery and follow-up phases, but not in the acute phase. Furthermore, among atopic patients, serum ECP levels were significantly higher in the recovery and follow-up phases than in the acute phase.
CONCLUSIONS
The present study demonstrated significant differences in eosinophil counts, serum IL-5, and serum ECP levels between atopic and non-atopic children with MP at admission, recovery, and 2 months after clinical recovery. These outcomes are suggestive of eosinophil-related hyperreactivity in atopic children, with this status maintained for at least 2 months after MP.
Keyword
MeSH Terms
Figure
Reference
-
1. Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008; 32:956–973.2. Clyde WA Jr. Clinical overview of typical Mycoplasma pneumoniae infections. Clin Infect Dis. 1993; 17:Suppl 1. S32–S36.3. Powell DA. Mycoplasmal infections. In : Kliegman RM, Stanton BF, St. Geme JW, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. Philadelphia (PA): Elsevier Saunders;2011. p. 1029–1032.4. Kim SH, Jung SW. Properties of M. pneumoniae infections in Korea, 2011. Public Health Wkly Rep. 2011; 4:893–896.5. Seggev JS, Lis I, Siman-Tov R, Gutman R, Abu-Samara H, Schey G, Naot Y. Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults. Ann Allergy. 1986; 57:263–265.6. Biscardi S, Lorrot M, Marc E, Moulin F, Boutonnat-Faucher B, Heilbronner C, Iniguez JL, Chaussain M, Nicand E, Raymond J, Gendrel D. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004; 38:1341–1346.7. Wilsher ML, Kolbe J. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med. 1995; 151:579–580.8. Biscione GL, Corne J, Chauhan AJ, Johnston SL. Increased frequency of detection of Chlamydophila pneumoniae in asthma. Eur Respir J. 2004; 24:745–749.9. Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002; 121:1782–1788.10. Hardy RD, Jafri HS, Olsen K, Hatfield J, Iglehart J, Rogers BB, Patel P, Cassell G, McCracken GH, Ramilo O. Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection-associated chronic reactive airway disease. Infect Immun. 2002; 70:649–654.11. Afshar R, Medoff BD, Luster AD. Allergic asthma: a tale of many T cells. Clin Exp Allergy. 2008; 38:1847–1857.12. Esposito S, Droghetti R, Bosis S, Claut L, Marchisio P, Principi N. Cytokine secretion in children with acute Mycoplasma pneumoniae infection and wheeze. Pediatr Pulmonol. 2002; 34:122–127.13. Jeong YC, Yeo MS, Kim JH, Lee HB, Oh JW. Mycoplasma pneumoniae infection affects the serum levels of vascular endothelial growth factor and interleukin-5 in atopic children. Allergy Asthma Immunol Res. 2012; 4:92–97.14. Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986; 39:177–253.15. Coyle AJ, Uchida D, Ackerman SJ, Mitzner W, Irvin CG. Role of cationic proteins in the airway. Hyperresponsiveness due to airway inflammation. Am J Respir Crit Care Med. 1994; 150:S63–S71.16. Kitahara H, Yamashita R, Kanemitsu T, Niwa I, Osano M. Relationship between M. pneumoniae infections and eosinophilia. Proceedings of the 12th Annual Congress of the Japanese Society of Mycoplasmology. Tokyo: Japanese Society of Mycoplasmology;1985. p. 75–78.17. Yamashita R, Kitahara H, Kanemitsu T, Takeda T, Yamaguchi S. Eosinophil cationic protein in the sera of patients with Mycoplasma pneumonia. Pediatr Infect Dis J. 1994; 13:379–381.18. Marsh DG, Bias WB, Ishizaka K. Genetic control of basal serum immunoglobulin E level and its effect on specific reaginic sensitivity. Proc Natl Acad Sci U S A. 1974; 71:3588–3592.19. Wittig HJ, Belloit J, De Fillippi I, Royal G. Age-related serum immunoglobulin E levels in healthy subjects and in patients with allergic disease. J Allergy Clin Immunol. 1980; 66:305–313.20. Peterson CG, Enander I, Nystrand J, Anderson AS, Nilsson L, Venge P. Radioimmunoassay of human eosinophil cationic protein (ECP) by an improved method. Establishment of normal levels in serum and turnover in vivo. Clin Exp Allergy. 1991; 21:561–567.21. MacDowell AL, Bacharier LB. Infectious triggers of asthma. Immunol Allergy Clin North Am. 2005; 25:45–66.22. Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, Gaydos CA, Martin RJ. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998; 158:998–1001.23. Chu HW, Rino JG, Wexler RB, Campbell K, Harbeck RJ, Martin RJ. Mycoplasma pneumoniae infection increases airway collagen deposition in a murine model of allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2005; 289:L125–L133.24. Martin RJ, Chu HW, Honour JM, Harbeck RJ. Airway inflammation and bronchial hyperresponsiveness after Mycoplasma pneumoniae infection in a murine model. Am J Respir Cell Mol Biol. 2001; 24:577–582.25. Choi IS, Byeon JH, Yoo Y, Lee KC, Choung JT. Increased serum interleukin-5 and vascular endothelial growth factor in children with acute mycoplasma pneumonia and wheeze. Pediatr Pulmonol. 2009; 44:423–428.26. Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003; 167:199–204.27. Nakajima H, Takatsu K. Role of cytokines in allergic airway inflammation. Int Arch Allergy Immunol. 2007; 142:265–273.28. Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992; 326:298–304.29. Frigas E, Gleich GJ. The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol. 1986; 77:527–537.30. Synek M, Beasley R, Frew AJ, Goulding D, Holloway L, Lampe FC, Roche WR, Holgate ST. Cellular infiltration of the airways in asthma of varying severity. Am J Respir Crit Care Med. 1996; 154:224–230.31. Oddera S, Silvestri M, Balbo A, Jovovich BO, Penna R, Crimi E, Rossi GA. Airway eosinophilic inflammation, epithelial damage, and bronchial hyperresponsiveness in patients with mild-moderate, stable asthma. Allergy. 1996; 51:100–107.32. Venge P. Monitoring the allergic inflammation. Allergy. 2004; 59:26–32.33. Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989; 142:4428–4434.34. Hernnäs J, Särnstrand B, Lindroth P, Peterson CG, Venge P, Malmström A. Eosinophil cationic protein alters proteoglycan metabolism in human lung fibroblast cultures. Eur J Cell Biol. 1992; 59:352–363.35. Löwhagen O, Wever AM, Lusuardi M, Moscato G, De Backer WA, Gandola L, Donner CF, Ahlstedt S, Larsson L, Holgate ST. The inflammatory marker serum eosinophil cationic protein (ECP) compared with PEF as a tool to decide inhaled corticosteroid dose in asthmatic patients. Respir Med. 2002; 96:95–101.36. Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol. 2002; 169:6459–6466.37. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000; 356:2144–2148.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study of Relationship between Bronchial Hyperreactivity(BHR) and Atopic Findings in Mycoplasma Pneumoniae Pneumonia
- Mycoplasma pneumoniae Infection Affects the Serum Levels of Vascular Endothelial Growth Factor and Interleukin-5 in Atopic Children
- A clinical study of mycoplasma pneumonia in children during recent 5 years
- Mycoplasma pneumoniae Pneumonia in Children
- Clinical Observation on Pneumonia due to Mycoplasma Pneumoniae in Children