J Clin Neurol.
2015 Jan;11(1):97-101. 10.3988/jcn.2015.11.1.97.
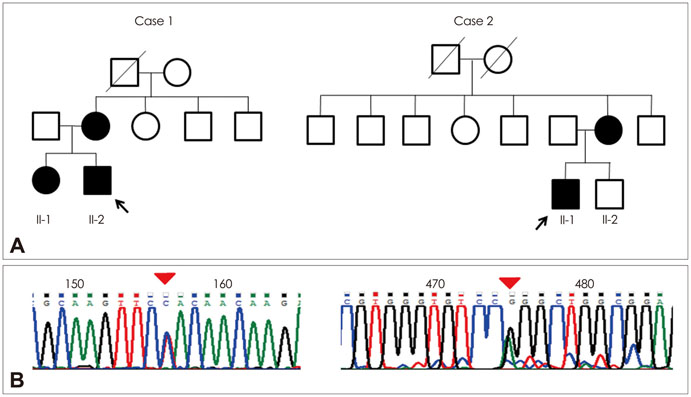
Mild Clinical Features and Histopathologically Atypical Cores in Two Korean Families with Central Core Disease Harboring RYR1 Mutations at the C-Terminal Region
- Affiliations
-
- 1Department of Neurology, Pusan National University Yangsan Hospital, Yangsan, Korea. dskim@pusan.ac.kr
- 2Department of Neurology, Pusan National University Hospital, Busan, Korea.
- 3Department of Pathology, Pusan National University Hospital, Busan, Korea.
- KMID: 2179580
- DOI: http://doi.org/10.3988/jcn.2015.11.1.97
Abstract
- BACKGROUND
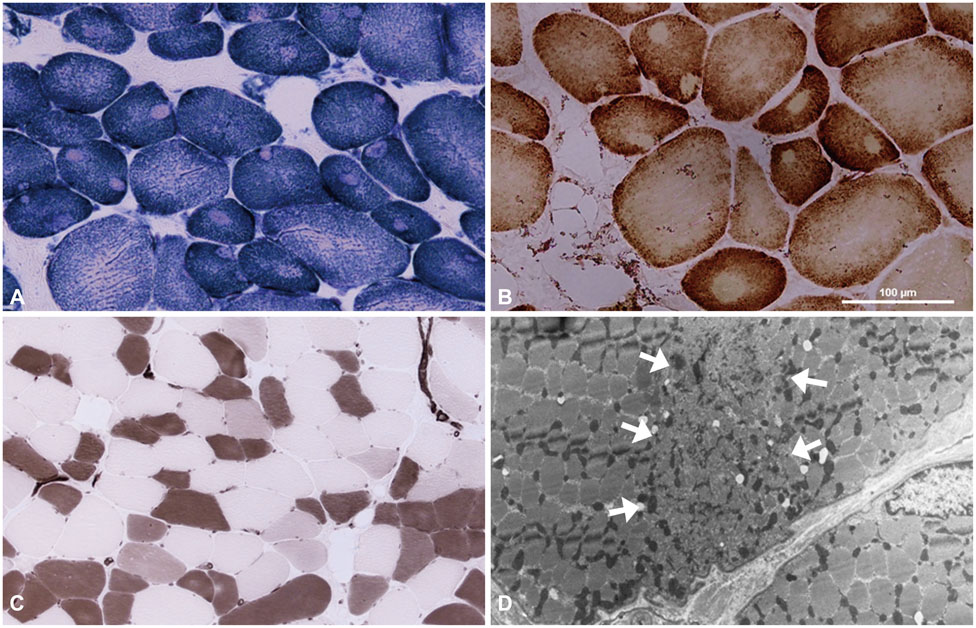
Central core disease (CCD) is a congenital myopathy characterized by distinctive cores in muscle fibers. Mutations in the gene encoding ryanodine receptor 1 (RYR1) have been identified in most CCD patients.
CASE REPORT
Two unrelated patients presented with slowly progressive or nonprogressive proximal muscle weakness since childhood. Their family history revealed some members with the same clinical problem. Histological analysis of muscle biopsy samples revealed numerous peripheral cores in the muscle fibers. RYR1 sequence analysis disclosed a novel mutation in exon 101 (c.14590T>C) and confirmed a previously reported mutation in exon 102 (c.14678G>A).
CONCLUSIONS
We report herein two families with CCD in whom missense mutations at the C-terminal of RYR1 were identified. Although it has been accepted that such mutations are usually associated with a severe clinical phenotype and clearly demarcated central cores, our patients exhibited a mild clinical phenotype without facial muscle involvement and skeletal deformities, and atypical cores in their muscle biopsy specimens.
MeSH Terms
Figure
Reference
-
1. Davis MR, Haan E, Jungbluth H, Sewry C, North K, Muntoni F, et al. Principal mutation hotspot for central core disease and related myopathies in the C-terminal transmembrane region of the RYR1 gene. Neuromuscul Disord. 2003; 13:151–157.
Article2. Romero NB, Monnier N, Viollet L, Cortey A, Chevallay M, Leroy JP, et al. Dominant and recessive central core disease associated with RYR1 mutations and fetal akinesia. Brain. 2003; 126(Pt 11):2341–2349.
Article3. Kossugue PM, Paim JF, Navarro MM, Silva HC, Pavanello RC, Gurgel-Giannetti J, et al. Central core disease due to recessive mutations in RYR1 gene: is it more common than described? Muscle Nerve. 2007; 35:670–674.
Article4. Monnier N, Romero NB, Lerale J, Nivoche Y, Qi D, MacLennan DH, et al. An autosomal dominant congenital myopathy with cores and rods is associated with a neomutation in the RYR1 gene encoding the skeletal muscle ryanodine receptor. Hum Mol Genet. 2000; 9:2599–2608.
Article5. Wu S, Ibarra MC, Malicdan MC, Murayama K, Ichihara Y, Kikuchi H, et al. Central core disease is due to RYR1 mutations in more than 90% of patients. Brain. 2006; 129(Pt 6):1470–1480.
Article6. Quinlivan RM, Muller CR, Davis M, Laing NG, Evans GA, Dwyer J, et al. Central core disease: clinical, pathological, and genetic features. Arch Dis Child. 2003; 88:1051–1055.
Article7. Sewry CA, Müller C, Davis M, Dwyer JS, Dove J, Evans G, et al. The spectrum of pathology in central core disease. Neuromuscul Disord. 2002; 12:930–938.
Article8. MacLennan DH, Duff C, Zorzato F, Fujii J, Phillips M, Korneluk RG, et al. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990; 343:559–561.
Article9. Lynch PJ, Tong J, Lehane M, Mallet A, Giblin L, Heffron JJ, et al. A mutation in the transmembrane/luminal domain of the ryanodine receptor is associated with abnormal Ca2+ release channel function and severe central core disease. Proc Natl Acad Sci U S A. 1999; 96:4164–4169.
Article10. Jungbluth H. Central core disease. Orphanet J Rare Dis. 2007; 2:25.
Article11. Zhou H, Jungbluth H, Sewry CA, Feng L, Bertini E, Bushby K, et al. Molecular mechanisms and phenotypic variation in RYR1-related congenital myopathies. Brain. 2007; 130(Pt 8):2024–2036.
Article12. Chang X, Jin Y, Zhao H, Huang Q, Wang J, Yuan Y, et al. Clinical features and ryanodine receptor type 1 gene mutation analysis in a Chinese family with central core disease. J Child Neurol. 2013; 28:384–388.
Article13. Balshaw D, Gao L, Meissner G. Luminal loop of the ryanodine receptor: a pore-forming segment? Proc Natl Acad Sci U S A. 1999; 96:3345–3347.
Article14. Tong J, McCarthy TV, MacLennan DH. Measurement of resting cytosolic Ca2+ concentrations and Ca2+ store size in HEK-293 cells transfected with malignant hyperthermia or central core disease mutant Ca2+ release channels. J Biol Chem. 1999; 274:693–702.
Article15. Dirksen RT, Avila G. Altered ryanodine receptor function in central core disease: leaky or uncoupled Ca(2+) release channels? Trends Cardiovasc Med. 2002; 12:189–197.
Article16. Tilgen N, Zorzato F, Halliger-Keller B, Muntoni F, Sewry C, Palmucci LM, et al. Identification of four novel mutations in the C-terminal membrane spanning domain of the ryanodine receptor 1: association with central core disease and alteration of calcium homeostasis. Hum Mol Genet. 2001; 10:2879–2887.
Article17. Loy RE, Orynbayev M, Xu L, Andronache Z, Apostol S, Zvaritch E, et al. Muscle weakness in Ryr1I4895T/WT knock-in mice as a result of reduced ryanodine receptor Ca2+ ion permeation and release from the sarcoplasmic reticulum. J Gen Physiol. 2011; 137:43–57.
Article18. Taylor A, Lachlan K, Manners RM, Lotery AJ. A study of a family with the skeletal muscle RYR1 mutation (c.7354C>T) associated with central core myopathy and malignant hyperthermia susceptibility. J Clin Neurosci. 2012; 19:65–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Genetic Analysis of the Ryanodine Receptor Gene (RYR1) in Korean Malignant Hyperthermia Families
- Clinical and Pathologic Findings of Korean Patients with RYR1-Related Congenital Myopathy
- A Double Mutation of the Ryanodine Receptor Type 1 Gene in a Malignant Hyperthermia Family with Multiminicore Myopathy
- Central core disease
- Characteristic Muscle Involvement on Magnetic Resonance Imaging in Central Core Disease