J Breast Cancer.
2015 Dec;18(4):371-377. 10.4048/jbc.2015.18.4.371.
Metastasis-Free Interval Is Closely Related to Tumor Characteristics and Has Prognostic Value in Breast Cancer Patients with Distant Relapse
- Affiliations
-
- 1Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. gsjjoon@yuhs.ac
- 2Biostatistics Collaboration Unit, Gangnam Medical Research Center, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Surgery, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- KMID: 2176286
- DOI: http://doi.org/10.4048/jbc.2015.18.4.371
Abstract
- PURPOSE
We investigated the relationships between metastasis-free interval (MFI) and tumor characteristics, and assessed the prognostic value of MFI for survival after metastasis in patients with metastatic breast cancer. Furthermore, we compared MFI among the subtypes.
METHODS
We identified 335 patients with postoperative tumor recurrence at distant site(s). All patients underwent curative resection and had a MFI of at least 6 months. MFI was categorized as short (<2 years), intermediate (> or =2 years and <5 years), or long (> or =5 years). Overall survival after metastasis (OSM) was estimated.
RESULTS
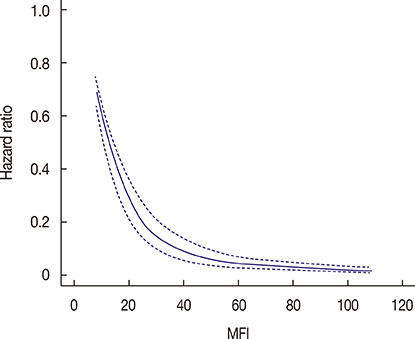
Patients with a shorter MFI were younger, more likely to have initial metastasis to visceral organs, and had a larger tumor with a higher stage and grade as well as a higher rate of nodal involvement at initial diagnosis. Among 136 patients with known disease subtypes, shorter MFI was associated with the triple-negative subtype while longer MFI was associated with the hormone receptor-positive/human epidermal growth factor receptor 2 negative subtype. Mortality after metastasis declined sharply with increasing MFI up to approximately 2 years, and continued gradually declining between 2 and 5 years. An MFI longer than 5 years did not add any survival benefit. MFI was a significant prognostic factor for OSM independent of nodal status, stage, metastatic site, and hormone receptor status of the metastasized cancer.
CONCLUSION
MFI is closely related to biological characteristics of both primary tumors and their metastases, and has a prognostic value for survival after metastasis. We therefore suggest investigation into treatments targeting improvement of MFI as a potential novel strategy.
Keyword
MeSH Terms
Figure
Reference
-
1. Insa A, Lluch A, Prosper F, Marugan I, Martinez-Agullo A, Garcia-Conde J. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999; 56:67–78.
Article2. Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008; 19:2012–2019.
Article3. Chang J, Clark GM, Allred DC, Mohsin S, Chamness G, Elledge RM. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer. 2003; 97:545–553.
Article4. Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012; 23:103–112.
Article5. Kwast AB, Voogd AC, Menke-Pluijmers MB, Linn SC, Sonke GS, Kiemeney LA, et al. Prognostic factors for survival in metastatic breast cancer by hormone receptor status. Breast Cancer Res Treat. 2014; 145:503–511.
Article6. Planchat E, Durando X, Abrial C, Thivat E, Mouret-Reynier MA, Ferriere JP, et al. Prognostic value of initial tumor parameters after metastatic relapse. Cancer Invest. 2011; 29:635–643.
Article7. Andre F, Bachelot T, Commo F, Campone M, Arnedos M, Dieras V, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 2014; 15:267–274.
Article8. Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013; 45:1439–1445.
Article9. Dignam JJ, Dukic V, Anderson SJ, Mamounas EP, Wickerham DL, Wolmark N. Hazard of recurrence and adjuvant treatment effects over time in lymph node-negative breast cancer. Breast Cancer Res Treat. 2009; 116:595–602.
Article10. Hilsenbeck SG, Ravdin PM, de Moor CA, Chamness GC, Osborne CK, Clark GM. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat. 1998; 52:227–237.
Article11. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996; 14:2738–2746.
Article12. Moeder CB, Giltnane JM, Harigopal M, Molinaro A, Robinson A, Gelmon K, et al. Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol. 2007; 25:5418–5425.
Article13. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989; 8:551–561.
Article14. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15:361–387.
Article15. James JJ, Evans AJ, Pinder SE, Gutteridge E, Cheung KL, Chan S, et al. Bone metastases from breast carcinoma: histopathological - radiological correlations and prognostic features. Br J Cancer. 2003; 89:660–665.
Article16. Colleoni M, O'Neill A, Goldhirsch A, Gelber RD, Bonetti M, Thurlimann B, et al. Identifying breast cancer patients at high risk for bone metastases. J Clin Oncol. 2000; 18:3925–3935.
Article17. Niikura N, Liu J, Hayashi N, Palla SL, Tokuda Y, Hortobagyi GN, et al. Treatment outcome and prognostic factors for patients with bone-only metastases of breast cancer: a single-institution retrospective analysis. Oncologist. 2011; 16:155–164.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Significance COX-2, VEGF and Cyclin D1 in Distant Metastasis of Breast Cancer
- The Prognostic Factors for Tumor Metastasis to Bone from Breast Cancer and Survival for Breast Cancer Patients after Bone Metastasis
- Survival from the First Recurrence and the Prognostic Factors of Patients with Recurrent Breast Cancer
- Prognostic Factors Influence on the Systemic Recurrence in Axillary Lymph Node Negative Breast Cancer
- Survival Analysis and Its Prognostic Factors after Distant Relapse in Breast Cancer Patients