J Breast Cancer.
2009 Dec;12(4):249-256. 10.4048/jbc.2009.12.4.249.
Expression of CXCR4 and SDF-1alpha in Primary Breast Cancers and Metastatic Lymph Nodes
- Affiliations
-
- 1Department of Pathology, The Catholic University of Korea, Daejeon St. Mary's Hospital, Daejeon, Korea.
- 2Department of Pathology, Chungnam National University, Daejeon, Korea. Qsong@cnu.ac.kr
- 3Department of Surgery, The Catholic University of Korea, Daejeon St. Mary's Hospital, Daejeon, Korea.
- KMID: 2175514
- DOI: http://doi.org/10.4048/jbc.2009.12.4.249
Abstract
- PURPOSE
A CXCR4/stroma derived factor-1alpha (SDF-1alpha, CXCL12) interaction is involved in many metastatic cancer mechanisms, including breast cancer. The primary objectives of this study were to investigate the correlation between CXCR4 and axillary lymph node metastasis and to clarify the interaction between CXCR4 in primary tumor cells and SDF-1alpha in metastatic lymph nodes. An analysis of the correlation between CXCR4, SDF-1alpha and clinicopathologic features was also performed. METHODS: Representative areas from 44 invasive ductal carcinomas were selected for construction of tissue microarrays using a 5 mm punch. Breast cancers (n=44), metastatic axillary lymph nodes (n=18) and non-metastatic axillary lymph nodes (n=26) were immunohistochemically stained for CXCR4, SDF-1alpha, estrogen receptor (ER), progesterone receptor (PR) and HER2. The parameters of age, tumor size, nuclear grade, histologic grade, lymph node status and pathologic node (pN) stage pN0 to pN3 were evaluated. RESULTS: CXCR4 expression was negatively correlated with increased age (p=0.005) and positively correlated with a large tumor size (p=0.043) and PR expression (p=0.027). CXCR4 expression was not correlated with metastatic lymph nodes (p=0.079) and SDF-1alpha expression in metastatic lymph nodes (p=0.062). However, CXCR4 nuclear positivity is correlated with lymph node metastasis (p=0.044). SDF-1alpha was not correlated with any clinicopathologic feature in a statistically significant manner. CONCLUSION: An evaluation of young age, large tumor size and PR expression helps predict lymph node metastasis and poor prognosis. Expression of CXCR4 nuclear positivity is correlated with a poor prognosis.
Keyword
MeSH Terms
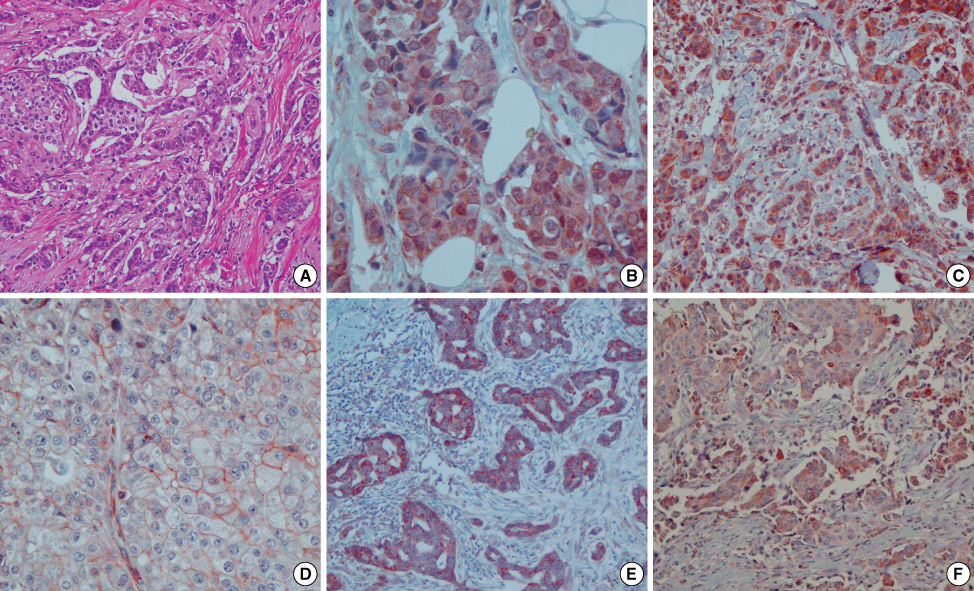
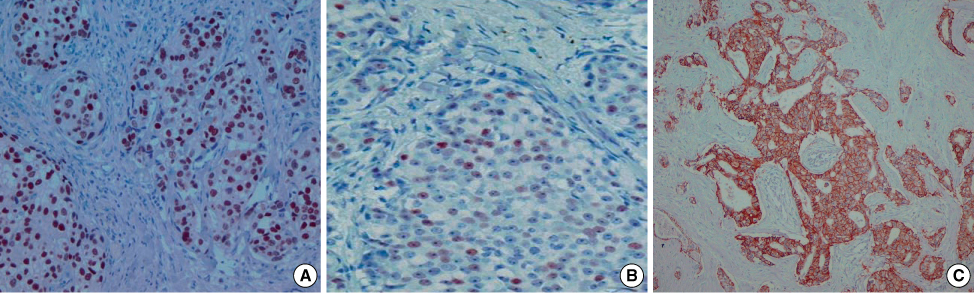
Figure
Cited by 1 articles
-
αB-Crystallin is a Novel Oncoprotein Associated with Poor Prognosis in Breast Cancer
Hae Sung Kim, Younok Lee, Young Ah Lim, Hee Joon Kang, Lee Su Kim
J Breast Cancer. 2011;14(1):14-19. doi: 10.4048/jbc.2011.14.1.14.
Reference
-
1. Nicolson GL. Paracrine and autocrine growth mechanisms in tumor metastasis to specific sites with particular emphasis on brain and lung metastasis. Cancer Metastasis Rev. 1993. 12:325–343.
Article2. Möhle R, Schittenhelm M, Failenschmid C, Bauts F, Kratz-Albers K, Serve H, et al. Functional response of leukaemic blasts to stromal cell-derived factor-1 correlates with preferential expression of the chemokine receptor CXCR4 in acute myelomonocytic and lymphoblastic leukaemia. Br J Haematol. 2000. 110:563–572.
Article3. Kang H, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The elevated level of CXCR4 is correlated with nodal metastasis of human breast cancer. Breast. 2005. 14:360–367.
Article4. Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, et al. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002. 62:6304–6311.5. Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004. 14:171–179.
Article6. Uchida D, Begum NM, Almofti A, Nakashiro K, Kawamata H, Tateishi Y, et al. Possible role of stromal-cell-derived factor-1/CXCR4 signaling on lymph node metastasis of oral squamous cell carcinoma. Exp Cell Res. 2003. 290:289–302.
Article7. Fernandis AZ, Prasad A, Band H, Klosel R, Ganju RK. Regulation of CXCR4 mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004. 23:157–167.
Article8. Pan J, Mestas J, Burdick MD, Phillips RJ, Thomas GV, Reckamp K, et al. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol Cancer. 2006. 5:56.
Article9. Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 2001. 61:4961–4965.10. Adams GB, Chabner KT, Foxall RB, Weibrecht KW, Rodrigues NP, Dombkowski D, et al. Heterologous cells cooperate to augment stem cell migration, homing, and engraftment. Blood. 2003. 101:45–51.
Article11. Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi M, Yamamoto N. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006. 60:273–276.
Article12. Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003. 89:462–473.
Article13. Geminder H, Sagi-Assif O, Goldberg L, Meshel T, Rechavi G, Witz IP, et al. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001. 167:4747–4757.
Article14. Helbig G, Christopherson KW 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003. 278:21631–21638.
Article15. Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005. 11:5686–5693.
Article16. Tsoli E, Tsantoulis PK, Papalambros A, Perunovic B, England D, Rawlands DA, et al. Simultaneous evaluation of maspin and CXCR4 in patients with breast cancer. J Clin Pathol. 2007. 60:261–266.
Article17. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999. 17:1474–1481.
Article18. Cabioglu N, Sahin A, Doucet M, Yavuz E, Igci A, Yildirim OE, et al. Chemokine receptor CXCR4 expression in breast cancer as a potential predictive marker of isolated tumor cells in bone marrow. Clin Exp Metastasis. 2005. 22:39–46.
Article19. Woo SU, Bae JW, Kim CH, Lee JB, Koo BW. Significant correlation between nuclear CXCR4 expression and axillary lymph node metastasis in hormonal receptor negative breast cancer. Ann Surg Oncol. 2008. 15:281–285.
Article20. Liu F, Lang R, Wei J, Fan Y, Cui L, Gu F, et al. Increased expression of SDF-1/CXCR4 is associated with lymph node metastasis of invasive micropapillary carcinoma of the breast. Histopathology. 2009. 54:741–750.
Article21. Su YC, Wu MT, Huang CJ, Hou MF, Yang SF, Chai CY. Expression of CXCR4 is associated with axillary lymph node status in patients with early breast cancer. Breast. 2006. 15:533–539.
Article22. Spano JP, Andre F, Morat L, Sabatier L, Besse B, Combadiere C, et al. Chemokine receptor CXCR4 and early stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004. 15:613–617.
Article23. Shim HS, Lau SK, Devi S, Yoon Y, Cho HT, Liang Z. Lower expression of CXCR4 in lymph node metastases than in primary breast cancers: Potential regulation by ligand-dependent degradation and HIF-1a. Biochem Biophys Res Commun. 2006. 346:252–258.
Article24. Wagner PL, Moo TA, Arora N, Liu YF, Zarnegar R, Scognamiglio T, et al. The chemokine receptors CXCR4 and CCR7 are associated with tumor size and pathologic indicators of tumor aggressiveness in papillary thyroid. Ann Surg Oncol. 2008. 15:2833–2841.
Article25. Song JS, Jung JK, Park JC, Kim DK, Jang SJ. Association of CXCR4 expression with metastasis and survival among patients with non-small cell lung cancer. Korean J Pathol. 2008. 42:358–364.26. Salvucci O, Bouchard A, Baccarelli A, Deschênes J, Sauter G, Simon R, et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006. 97:275–283.
Article27. Bae YK, Gong GY, Kang J, Lee AW, Cho EY, Lee JS, et al. Estrogen receptor expression in Korean breast carcinoma and comparison of three anti-ER antibodies. 2009. In : 61th fall annual meeting of Korean Society of Pathologists; –43. abstract #4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stromal-cell-derived Factor 1-alpha Promotes Tumor Progression in Colorectal Cancer
- Production of Stromal Cell-Derived Factor-1 (SDF-1)and Expression of CXCR4 in Human Bone Marrow Endothelial Cells
- Correlation between p53 and MIB1 Index Expression of Primary Tumor and Metastatic Lymph Node in Breast Cancer
- Correlation between p53 and MIB1 Index Expression of Primary Tumor and Metastatic Lymph Node in Breast Cancer
- Silver-Enhanced In Situ Hybridization as an Alternative to Fluorescence In Situ Hybridization for Assaying HER2 Amplification in Clinical Breast Cancer