Safety and Efficacy of Adalimumab for Patients With Moderate to Severe Crohn's Disease: The Taiwan Society of Inflammatory Bowel Disease (TSIBD) Study
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Mackay Memorial Hospital, Taipei, Taiwan.
- 2Mackay Junior College of Medicine, Nursing and Management, Taipei, Taiwan.
- 3Mackay Medical College, New Taipei, Taiwan. jmwong@ntu.edu.tw
- 4Department of Internal Medicine, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan.
- 5Division of Gastroenterology and Hepatology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan.
- 6Division of Colon and Rectal Surgery, Department of Surgery, Mackay Memorial Hospital, Taipei, Taiwan.
- 7Department of Internal Medicine, Medical College and Hospital, National Cheng-Kung University, Tainan, Taiwan.
- 8Division of Gastroenterology, Chung Shan Medical University, Taichung, Taiwan.
- 9Department of Gastroenterology, Kaohsiung Medical University, Kaohsiung, Taiwan.
- 10Department of Gastroenterology, Changhua Christian Hospital, Changhua, Taiwan.
- 11Division of Colon and Rectal Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei, Taiwan.
- 12Department of Surgery, School of Medicine, National Yang-Ming University, Taipei, Taiwan.
- 13Division of Gastroenterology, Department of Medicine, Show Chwan Memorial Hospital, Changhuan, Taiwan.
- 14Department of Pediatrics, National Taiwan University, Taipei, Taiwan.
- KMID: 2174405
- DOI: http://doi.org/10.5217/ir.2014.12.4.287
Abstract
- BACKGROUND/AIMS
Only moderate to severe Crohn's Disease (CD) patients without a satisfactory conventional therapy effect are eligible to get reimbursement from the National Health Insurance of Taiwan for using adalimumab. These are more stringent criteria than in many Western countries and Japan and Korea. We aim to explore the efficacy of using adalimumab in CD patients under such stringent criteria.
METHODS
A retrospective analysis was conducted in nine medical centers in Taiwan and we collected the results of CD patients receiving adalimumab from Sep 2009 to Mar 2014. The clinical characteristics, response measured by CDAI (Crohn's Disease Activity Index), adverse events and survival status were recorded and analyzed. CR-70, CR-100, and CR-150 were defined as attaining a CDAI decrease of 70, 100 or 150 points compared with baseline.
RESULTS
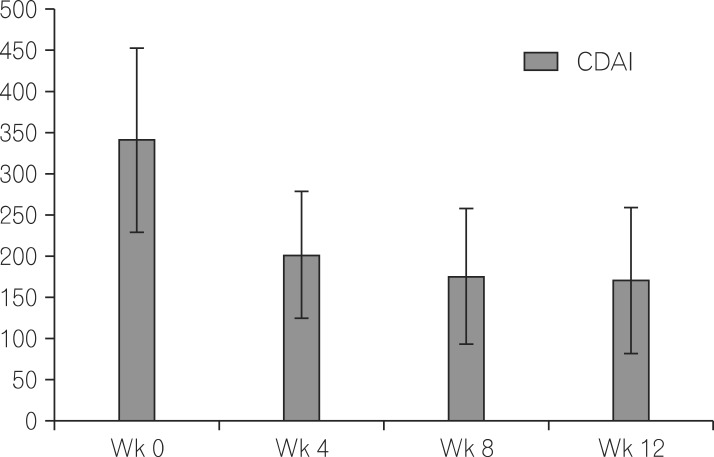
A total of 103 CD patient records were used in this study. Sixty percent of these patients received combination therapy of adalimumab together with immunomodulators. CR-70 was 68.7%, 74.5% and 88.4% after week 4, 8 and 12 of treatment, respectively. The steroid-free rate, complications and survival were 47.6%, 9.7% and 99% of patients, respectively. In considering the mucosal healing, only 25% patients achieve mucosal healing after treatment for 6 to12 months. Surgery was still needed in 16.5% of patients. Combination treatment of adalimumab with immunomodulators further decreased the level of CDAI at week 8 when compared with the monotherapy.
CONCLUSIONS
Even under the stringent criteria for using adalimumab, the response rate was comparable to those without stringent criteria.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Balloon-Assisted Enteroscopy and Capsule Endoscopy in Suspected Small Bowel Crohn’s Disease
Hsu-Heng Yen, Chen-Wang Chang, Jen-Wei Chou, Shu-Chen Wei
Clin Endosc. 2017;50(5):417-423. doi: 10.5946/ce.2017.142.Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide populationbased study
Hsu-Heng Yen, Meng-Tzu Weng, Chien-Chih Tung, Yu-Ting Wang, Yuan Ting Chang, Chin-Hao Chang, Ming-Jium Shieh, Jau-Min Wong, Shu-Chen Wei
Intest Res. 2019;17(1):54-62. doi: 10.5217/ir.2018.00096.Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment
Dong Il Park, Tadakazu Hisamatsu, Minhu Chen, Siew Chien Ng, Choon Jin Ooi, Shu Chen Wei, Rupa Banerjee, Ida Normiha Hilmi, Yoon Tae Jeen, Dong Soo Han, Hyo Jong Kim, Zhihua Ran, Kaichun Wu, Jiaming Qian, Pin-Jin Hu, Katsuyoshi Matsuoka, Akira Andoh, Yasuo Suzuki, Kentaro Sugano, Mamoru Watanabe, Toshifumi Hibi, Amarender S. Puri, Suk-Kyun Yang
Intest Res. 2018;16(1):4-16. doi: 10.5217/ir.2018.16.1.4.High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India
Ashish Agarwal, Saurabh Kedia, Saransh Jain, Vipin Gupta, Sawan Bopanna, Dawesh P Yadav, Sandeep Goyal, Venigalla Pratap Mouli, Rajan Dhingra, Govind Makharia, Vineet Ahuja
Intest Res. 2018;16(4):588-598. doi: 10.5217/ir.2018.00023.
Reference
-
1. Sung JJ, Kamm MA, Marteau P. Asian perspectives in the management of inflammatory bowel disease: findings from a recent survey. J Gastroenterol Hepatol. 2010; 25:183–193. PMID: 19929931.
Article2. Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease - current knowledge and future perspectives. J Crohns Colitis. 2008; 2:279–290. PMID: 21172225.
Article3. Tursi A, Elisei W, Picchio M, et al. Effectiveness and safety of infliximab and adalimumab for ambulatory Crohn's disease patients in primary gastroenterology centres. Eur J Intern Med. 2014; 25:485–490. PMID: 24631020.
Article4. Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012; 142:1102–1111. PMID: 22326435.
Article5. Peters CP, Eshuis EJ, Toxopeus FM, et al. Adalimumab for Crohn's disease: long-term sustained benefit in a population-based cohort of 438 patients. J Crohns Colitis. 2014; 8:866–875. PMID: 24491515.
Article6. Teriaky A, Gregor J, Yan B, Ponich T, Chande N, Mosli M. The safety and efficacy of adalimumab in patients with Crohn's disease: the experience of a single Canadian tertiary care centre. Scand J Gastroenterol. 2014; 49:280–286. PMID: 24329040.
Article7. Asgharpour A, Cheng J, Bickston SJ. Adalimumab treatment in Crohn's disease: an overview of long-term efficacy and safety in light of the EXTEND trial. Clin Exp Gastroenterol. 2013; 6:153–160. PMID: 24039442.8. Zorzi F, Zuzzi S, Onali S, et al. Efficacy and safety of infliximab and adalimumab in Crohn's disease: a single centre study. Aliment Pharmacol Ther. 2012; 35:1397–1407. PMID: 22519466.
Article9. Lichtenstein GR, Panaccione R, Mallarkey G. Efficacy and safety of adalimumab in Crohn's disease. Therap Adv Gastroenterol. 2008; 1:43–50.10. Seiderer J, Brand S, Dambacher J, et al. Adalimumab in patients with Crohn's disease--safety and efficacy in an open-label single centre study. Aliment Pharmacol Ther. 2007; 25:787–796. PMID: 17373917.
Article11. Hinojosa J, Gomollon F, Garcia S, et al. Efficacy and safety of short-term adalimumab treatment in patients with active Crohn's disease who lost response or showed intolerance to infliximab: a prospective, open-label, multicentre trial. Aliment Pharmacol Ther. 2007; 25:409–418. PMID: 17269996.
Article12. Ueno F, Matsui T, Matsumoto T, Matsuoka K, Watanabe M, Hibi T. Evidence-based clinical practice guidelines for Crohn's disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013; 48:31–72. PMID: 23090001.
Article13. Thia KT, Loftus EV Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008; 103:3167–3182. PMID: 19086963.
Article14. Wei SC, Lin MH, Tung CC, et al. A nationwide population-based study of the inflammatory bowel diseases between 1998 and 2008 in Taiwan. BMC Gastroenterol. 2013; 13:166. PMID: 24314308.15. Sostegni R, Daperno M, Scaglione N, Lavagna A, Rocca R, Pera A. Review article: Crohn's disease: monitoring disease activity. Aliment Pharmacol Ther. 2003; 17(Suppl 2):11–17. PMID: 12786607.16. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006; 130:323–333. PMID: 16472588.17. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007; 132:52–65. PMID: 17241859.18. Ishida K, Inoue T, Fujiwara K, et al. Clinical effects of adalimumab treatment with concomitant azathioprine in Japanese Crohn's disease patients. World J Gastroenterol. 2013; 19:2676–2682. PMID: 23674875.
Article19. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395. PMID: 20393175.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Seminar Report From the 2014 Taiwan Society of Inflammatory Bowel Disease (TSIBD) Spring Forum (May 24th, 2014): Crohn's Disease Versus Intestinal Tuberculosis Infection
- Clinical efficacy of adalimumab versus infliximab and the factors associated with recurrence or aggravation during treatment of anal fistulas in Crohn's disease
- Pulmonary Extraintestinal Manifestation of Crohn's Disease Treated Successfully with Adalimumab
- Adalimumab Treatment in Pediatric-Onset Crohn's Disease Patients after Infliximab Failure: A Single Center Study
- An unusual cause of distal duodenal bleeding